SLOS to be assessed: SLO1: Interpret, generate, and practice practical application of graphical and/or algebraic relationships between material structure, composition, properties, and process variables such as stress-strain graphs and phase diagrams. Complete the following hands-on activity involving the construction of the traditional unit cells of the simple cubic, body centered cubic, and face centered cubic Bravais lattice types. 1) Please create models of simple cubic, FCC, and BCC crystal structures using a common uniform spherical objects that can be easily cut such as styrofoam balls (available from craft stores such as Michaels or Hobby Lobby or even round fruit. You can use toothpicks or low temperature hot glue to hold the parts together. For each structure (simple cubic, body centered cubic, or face-centered cubic), include a picture of the dismantled unit cell to demonstrate the number of atoms/unit cell and a picture of your unit cell. The corners of all the unit cells should consist of 1/8 spheres. 2) Verify the relationships between the lattice parameter and atomic radii shown in the lecture slides for each unit cell using the table below. Write a conclusion based on your results.
SLOS to be assessed: SLO1: Interpret, generate, and practice practical application of graphical and/or algebraic relationships between material structure, composition, properties, and process variables such as stress-strain graphs and phase diagrams. Complete the following hands-on activity involving the construction of the traditional unit cells of the simple cubic, body centered cubic, and face centered cubic Bravais lattice types. 1) Please create models of simple cubic, FCC, and BCC crystal structures using a common uniform spherical objects that can be easily cut such as styrofoam balls (available from craft stores such as Michaels or Hobby Lobby or even round fruit. You can use toothpicks or low temperature hot glue to hold the parts together. For each structure (simple cubic, body centered cubic, or face-centered cubic), include a picture of the dismantled unit cell to demonstrate the number of atoms/unit cell and a picture of your unit cell. The corners of all the unit cells should consist of 1/8 spheres. 2) Verify the relationships between the lattice parameter and atomic radii shown in the lecture slides for each unit cell using the table below. Write a conclusion based on your results.
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
The project has already been completed, just go through it and confirm if the measurements and picture provided fits the description and specified measurements, PLEASE DRAW THE STRUCTURES PER SPECIFICATIONS BOTH DISMANTELED AND CONSTRUCTED!!!
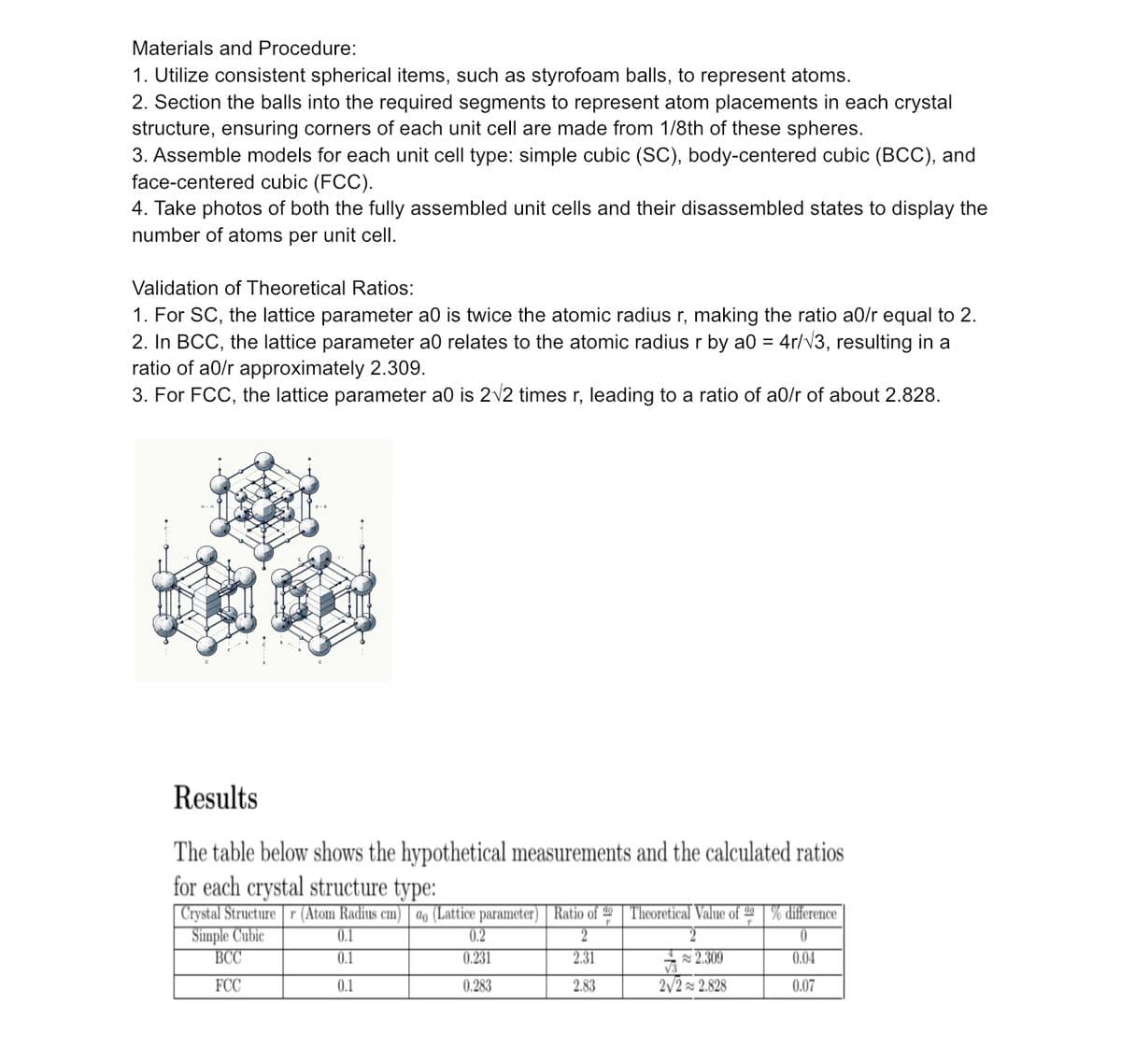
Transcribed Image Text:Materials and Procedure:
1. Utilize consistent spherical items, such as styrofoam balls, to represent atoms.
2. Section the balls into the required segments to represent atom placements in each crystal
structure, ensuring corners of each unit cell are made from 1/8th of these spheres.
3. Assemble models for each unit cell type: simple cubic (SC), body-centered cubic (BCC), and
face-centered cubic (FCC).
4. Take photos of both the fully assembled unit cells and their disassembled states to display the
number of atoms per unit cell.
Validation of Theoretical Ratios:
1. For SC, the lattice parameter a0 is twice the atomic radius r, making the ratio a0/r equal to 2.
2. In BCC, the lattice parameter a0 relates to the atomic radius r by a0 = 4r/√3, resulting in a
ratio of a0/r approximately 2.309.
3. For FCC, the lattice parameter a0 is 2√2 times r, leading to a ratio of a0/r of about 2.828.
Results
The table below shows the hypothetical measurements and the calculated ratios
for each crystal structure type:
Crystal Structurer (Atom Radius cm) ao (Lattice parameter) Ratio of 49 Theoretical Value of 49% difference
Simple Cubic
0.1
2
0
BCC
0.1
2.31
0.04
FCC
0.1
2.83
0.07
0.2
0.231
0.283
2.309
2√2 2.828
Transcribed Image Text:SLOS to be assessed:
SLO1: Interpret, generate, and practice practical application of graphical and/or algebraic relationships
between material structure, composition, properties, and process variables such as stress-strain graphs
and phase diagrams.
Complete the following hands-on activity involving the construction of the traditional unit
cells of the simple cubic, body centered cubic, and face centered cubic Bravais lattice types.
1) Please create models of simple cubic, FCC, and BCC crystal structures using a common
uniform spherical objects that can be easily cut such as styrofoam balls (available from craft
stores such as Michaels or Hobby Lobby or even round fruit. You can use toothpicks
or low temperature hot glue to hold the parts together. For each structure (simple cubic, body
centered cubic, or face-centered cubic), include a picture of the dismantled unit cell to
demonstrate the number of atoms/unit cell and a picture of your unit cell. The corners of all
the unit cells should consist of 1/8 spheres.
2) Verify the relationships between the lattice parameter and atomic radii shown in the
lecture slides for each unit cell using the table below. Write a conclusion based on your
results.
Crystal
Structure
Simple Cubic
BCC
FCC
r=Atom
Radius (cm)
ao (lattice parameter)
Ratio of
ao/r
Theoretical
Value of
a./r
%
difference
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY