Sodium hydrogen carbonate (NaHCO,), also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. Acid indigestion is the burning sensation you get in your stomach when it contains too much hydrochloric acid (HCI), which the stomach secretes to help digest food, Drinking a glass of water containing dissolved NaHCO, neutralizes excess HCl through this reaction: HCl(@q) + NaHCO,(0g) – NaCl(q) + H+O) + CO,(9) The CO, gas produced is what makes you burp after drinking the solution. Suppose the fluid in the stomach of a woman suffering from indigestion can be considered to be 100. mL of a 0.050 M HCl solution. What mass of NaHCO, would she need to ingest to neutralize this much HCl ? Be sure your answer has the correct number of significant digits. 0. alb M
Sodium hydrogen carbonate (NaHCO,), also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. Acid indigestion is the burning sensation you get in your stomach when it contains too much hydrochloric acid (HCI), which the stomach secretes to help digest food, Drinking a glass of water containing dissolved NaHCO, neutralizes excess HCl through this reaction: HCl(@q) + NaHCO,(0g) – NaCl(q) + H+O) + CO,(9) The CO, gas produced is what makes you burp after drinking the solution. Suppose the fluid in the stomach of a woman suffering from indigestion can be considered to be 100. mL of a 0.050 M HCl solution. What mass of NaHCO, would she need to ingest to neutralize this much HCl ? Be sure your answer has the correct number of significant digits. 0. alb M
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter16: Solutions
Section: Chapter Questions
Problem 100E: Potassium hydrogen phthalate is a solid, monoprotic acid frequently used in the laboratory as a...
Related questions
Question
please make sure correct sig figs!!!
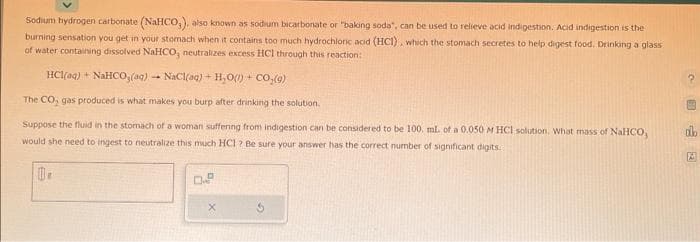
Transcribed Image Text:Sodium hydrogen carbonate (NaHCO,), also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. Acid indigestion is the
burning sensation you get in your stomach when it contains too much hydrochloric acid (HCI), which the stomach secretes to help digest food, Drinking a glass
of water containing dissolved NaHCO, neutralizes excess HCl through this reaction:
HC1(@q) + NaHCO,(g) – NaCl(q) + H+O) + CO,(9)
The CO, gas produced is what makes you burp after drinking the solution.
Suppose the fluid in the stomach of a woman suffering from indigestion can be considered to be 100. mL of a 0.050 M HCl solution. What mass of NaHCO,
would she need to ingest to neutralize this much HCl ? Be sure your answer has the correct number of significant digits.
10.
0.9
?
alb
M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning