Solid lead(II) nitrate is slowly added to 150 mL of a 0.106 M potassium carbonate solution until the concentration of lead ion is 0.0358 M. The percent of carbonate ion remaining in solution is Submit Answer Retry Entire Group 6 more group attempts remaining
Solid lead(II) nitrate is slowly added to 150 mL of a 0.106 M potassium carbonate solution until the concentration of lead ion is 0.0358 M. The percent of carbonate ion remaining in solution is Submit Answer Retry Entire Group 6 more group attempts remaining
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter11: Solutions
Section: Chapter Questions
Problem 39P
Related questions
Question
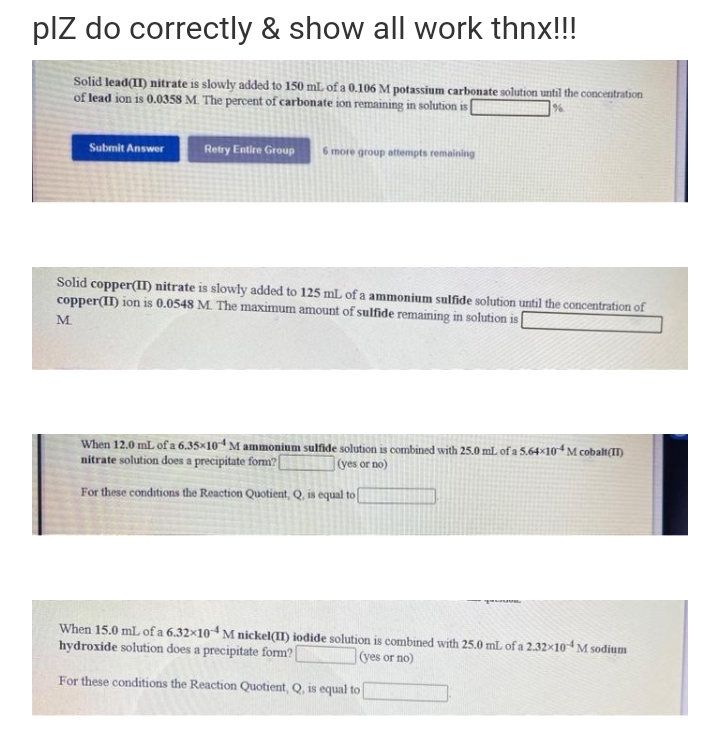
Transcribed Image Text:plz do correctly & show
all work thnx!!!
Solid lead(II) nitrate is slowly added to 150 mL of a 0.106 M potassium carbonate solution until the concentration
of lead ion is 0.0358 M. The percent of carbonate ion remaining in solution is [
Submit Answer
Retry Entire Group
6 more group attempts remaining
Solid copper(II) nitrate is slowly added to 125 mL of a ammonium sulfide solution until the concentration of
copper(II) ion is 0.0548 M. The maximum amount of sulfide remaining in solution is
M.
When 12.0 mL of a 6.35×104 M ammonium sulfide solution is combined with 25.0 mL of a 5.64×104M cobalt(II)
nitrate solution does a precipitate form?
(yes or no)
For these conditions the Reaction Quotient, Q, is equal to[
When 15.0 mL of a 6.32x104 M nickel(II) iodide solution is combined with 25.0 mL of a 2.32×104M sodium
hydroxide solution does a precipitate form?
(yes or no)
For these conditions the Reaction Quotient, Q, is equal to
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning