Solid sodium bicarbonate reacted with aqueous Hydrochloric acid(hydrogen monochloride) to form aqueous sodium chloride, carbon dioxide gas, and liquid water.
Solid sodium bicarbonate reacted with aqueous Hydrochloric acid(hydrogen monochloride) to form aqueous sodium chloride, carbon dioxide gas, and liquid water.
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 52E: Before the introduction of chlorofluorocarbons, sulfur dioxide (enthalpy of vaporization, 6.00...
Related questions
Question
Question in image
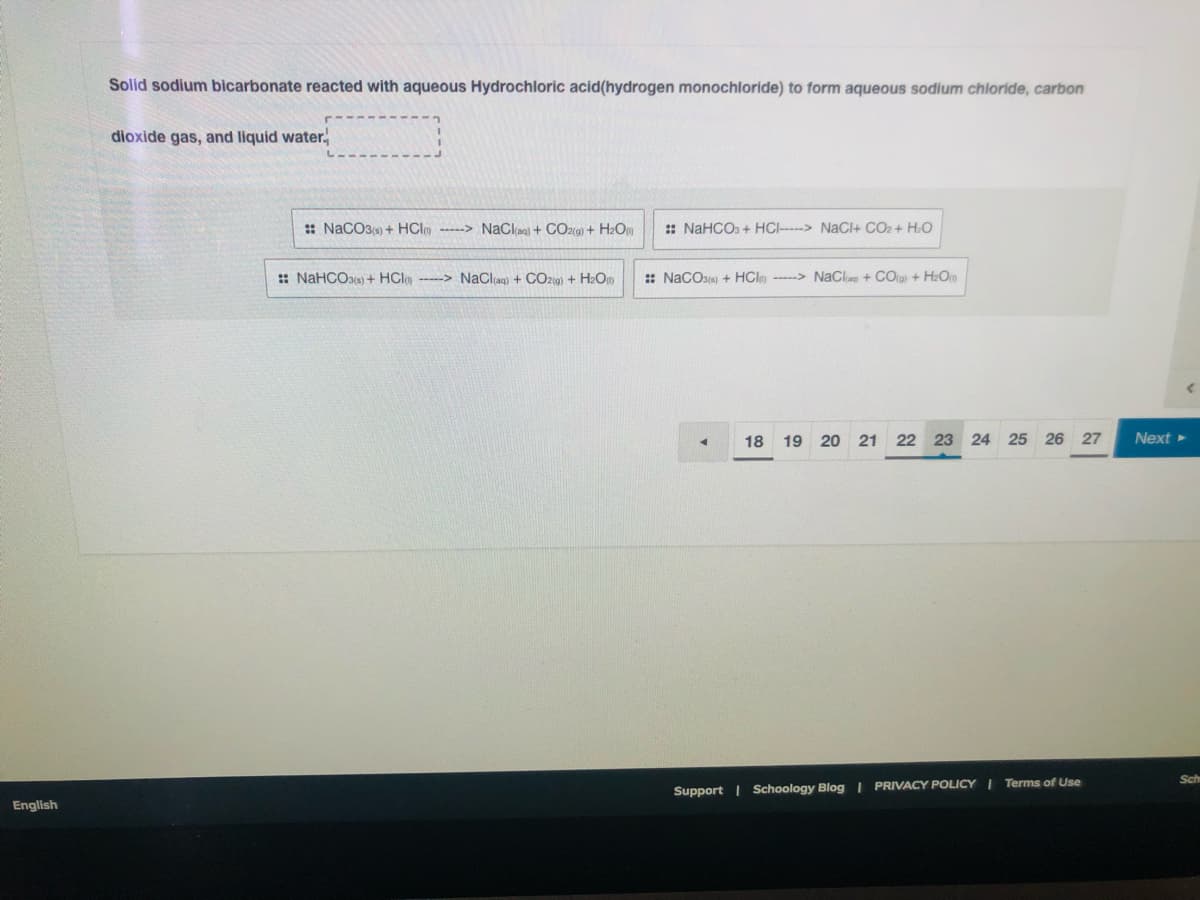
Transcribed Image Text:Solid sodium bicarbonate reacted with aqueous Hydrochloric acid(hydrogen monochloride) to form aqueous sodium chloride, carbon
dioxide gas, and liquid water.,
: NaCO3(e) + HClm ---> NaClag) + CO2(g) + H2O
: NAHCO. + HCI----> NaCl+ CO2+ H.O
:: NaHCOae) + HClg ----> NaClag) + CO219) + H2Op
:: NaCOse) + HClm -----> NaCla + COrg + H2O
18
19 20 21 22 23 24 25 26 27
Next
Sch
Support | Schoology Blog | PRIVACY POLICY I Terms of Use
English
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning