Solve the following problems and show complete solutions. The final answers must be reported in proper units and number of significant figures. Assume that all solutions were prepared at 25°C, and the solutes were completely dissolved. Molar masses should be based on the Periodic Table provided. Density values are provided if needed. In the lead content analysis of a wastewater sample, 50.00 mL of the original sample was taken and diluted with 150.00 mL deionized water. The diluted sample was found to have a lead content of 17.8 ppb. What is the lead content (in ppb) of the original wastewater sample? DENSITY VALUES AT 25°C Ethanol: 0.789 g/mL Acetic Acid: 1.05 g/mL Water: 0.997 g/mL
Solve the following problems and show complete solutions. The final answers must be reported in proper units and number of significant figures. Assume that all solutions were prepared at 25°C, and the solutes were completely dissolved. Molar masses should be based on the Periodic Table provided. Density values are provided if needed. In the lead content analysis of a wastewater sample, 50.00 mL of the original sample was taken and diluted with 150.00 mL deionized water. The diluted sample was found to have a lead content of 17.8 ppb. What is the lead content (in ppb) of the original wastewater sample? DENSITY VALUES AT 25°C Ethanol: 0.789 g/mL Acetic Acid: 1.05 g/mL Water: 0.997 g/mL
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter8: Solutions
Section: Chapter Questions
Problem 8.5EP: For each of the following pairs of solutions, select the solution for which solute solubility is...
Related questions
Question
5.
Kindly solve for me to understand.
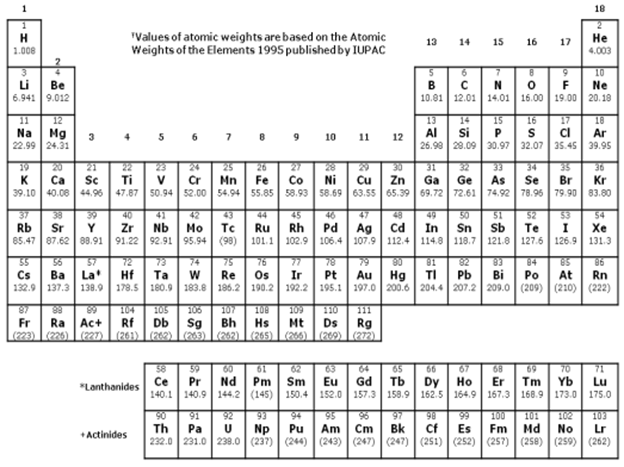
Transcribed Image Text:18
2
"Values of atomic weights are based on the Atomic
Weights of the Elements 1995 published by IUPAC
Не
13
14
15
16
17
1.008
4.003
10
Li
Be
6.941 9.012
B
10.81 12.01
F
Ne
19.00 20.18
14.01
16.00
11
12
13
14
15
16
17
18
Na Mg
3
6.
10
11
12
Al
Si
ci
Ar
22.99 24.31
26.98 28.09 30.97 32.07 35.45 39.95
22
23
24
25
Mn
27
28
29
30
20
Са
39.10 40.06 44.96
TE
32
Ge
EE
As
ÞE
35
Br
19
21
26
36
Sc
Ti
47.87 50.94 52.00 54.94 55.85 58.93 58.69 63.55 65.39 69.72 72.61 74.92 78.96 79.90 83.80
V
Čr
Fe
Co
Ni
Cu
Zn
Ga
Se
Kr
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
$3
54
Zr
85.47 87.62 88.91 91.22 92.91 95.94
Ru
To
(98)
101.1 102.9 106.4 107.9 112.4 114.8 118.7 121.8 127.6 126.9 131.3
Xe
Rb
Sr
Nb
Mo
Rh
Pd
Ag
Cd
In
Sn
Sb
Te
78
81 2 83
Pb
65
At
55
56
57
72
Hf
178.5 180.9 183.8 186.2 190.2| 192.2 195.1
73
Ta
74
75
76
77
79
80
84
86
Cs
Ва
La
Re
Os
Ir
Pt
Au
197.0 200.6 204.4 207.2 209.0 (209) (210) | (222)
Hg
TI
Bị
Po
Rn
132.9 137.3 138.9
97
Fr
228) (226) (227) (261) (262) (263) (262) (265) (266) (260) (272)
104
105
Db
106
107
108
109
Mt
110
111
Ra
Ac+
Rf
Sg
Bh
Hs
Ds
Rg
64
Gd
140.1 140.9 144.2 (145) 150.4 152.0 157.3 158.9 162.5 164.9 167.3 168.9 173.0 175.0
59
Pr
60
Nd
61
Pm
62
Sm
63
Eu
65
Tb
67
68
Er
69
Tm
70
58
Ce
71
Lu
66
*Lanthanides
Dy Ho
Yb
97
Bk
232.0 231.0 238.0 (237) | (244) (243) (247) (247) (251) (252) (257) (258) (259) (262)
91
92
93
94
Pu
95
Am
98
Th Pa
99
Es
100
Fm
101
Md
102
No
103
Lr
Np
Cm
cf
*Actinides
9.
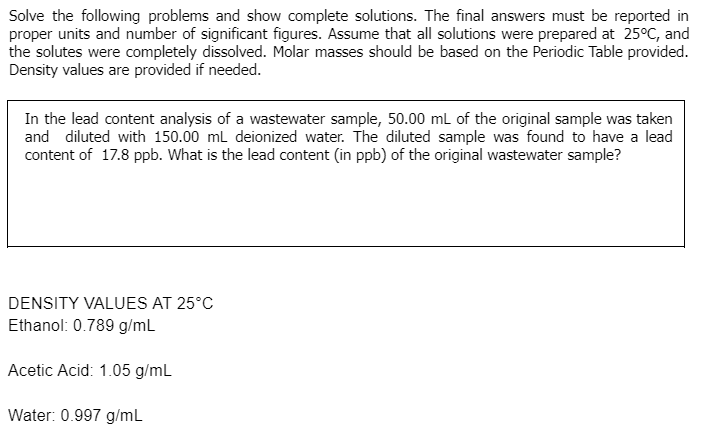
Transcribed Image Text:Solve the following problems and show complete solutions. The final answers must be reported in
proper units and number of significant figures. Assume that all solutions were prepared at 25°C, and
the solutes were completely dissolved. Molar masses should be based on the Periodic Table provided.
Density values are provided if needed.
In the lead content analysis of a wastewater sample, 50.00 ml of the original sample was taken
and diluted with 150.00 ml deionized water. The diluted sample was found to have a lead
content of 17.8 ppb. What is the lead content (in ppb) of the original wastewater sample?
DENSITY VALUES AT 25°C
Ethanol: 0.789 g/ml
Acetic Acid: 1.05 g/mL
Water: 0.997 g/mL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
