Sometimes in lab we collect the gas formed by a chemical reaction over water (see sketch at right). This makes it easy to isolate and measure the amount of gas produced. Suppose the CO₂ gas evolved by a certain chemical reaction taking place at 50.0 °C is collected over water, using an apparatus something like that in the sketch, and the final volume of gas in the collection tube is measured to be 37.4 mL. gn collected X gas water chemical Sketch of a gas-collection apparatus Calculate the mass of CO, that is in the collection tube. Round your answer to 2 significant digits. You can make any normal and reasonable assumption about the reaction conditions and the nature of the gases. reaction
Sometimes in lab we collect the gas formed by a chemical reaction over water (see sketch at right). This makes it easy to isolate and measure the amount of gas produced. Suppose the CO₂ gas evolved by a certain chemical reaction taking place at 50.0 °C is collected over water, using an apparatus something like that in the sketch, and the final volume of gas in the collection tube is measured to be 37.4 mL. gn collected X gas water chemical Sketch of a gas-collection apparatus Calculate the mass of CO, that is in the collection tube. Round your answer to 2 significant digits. You can make any normal and reasonable assumption about the reaction conditions and the nature of the gases. reaction
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter5: The Gaseous State
Section: Chapter Questions
Problem 5.127QP: A 1.000-g sample of an unknown gas at 0C gives the following data: P(atm) V (L) 0.2500 3.1908 0.5000...
Related questions
Question
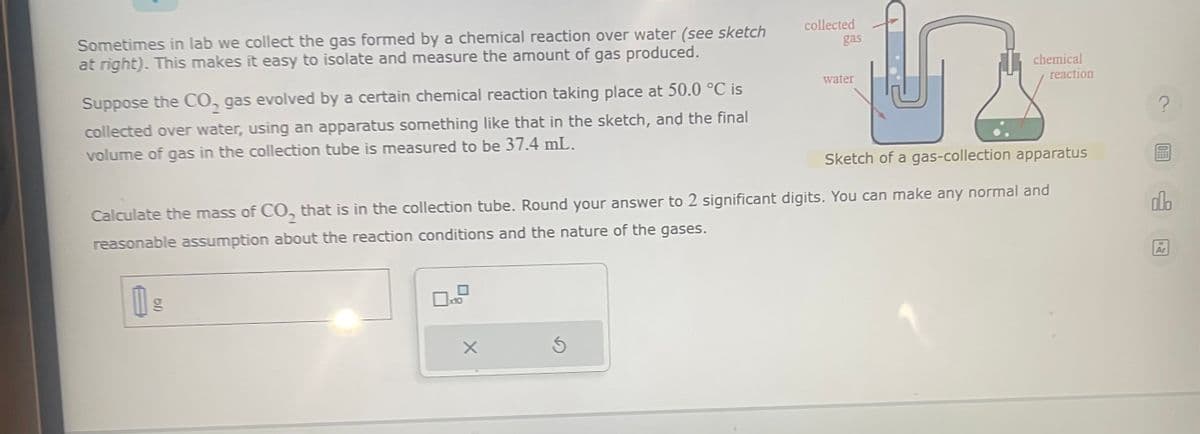
Transcribed Image Text:Sometimes in lab we collect the gas formed by a chemical reaction over water (see sketch
at right). This makes it easy to isolate and measure the amount of gas produced.
Suppose the CO₂, gas evolved by a certain chemical reaction taking place at 50.0 °C is
collected over water, using an apparatus something like that in the sketch, and the final
volume of gas in the collection tube is measured to be 37.4 mL.
X
$₁
Sketch of a gas-collection apparatus
S
collected
gas
water
Calculate the mass of CO, that is in the collection tube. Round your answer to 2 significant digits. You can make any normal and
reasonable assumption about the reaction conditions and the nature of the gases.
19
chemical
reaction
?
do
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning