Specify both the alcohol starting material and the reagents you would use in each step in a synthesis of the compound shown. If the synthesis requires only two steps enter "none" for step 3. CI Alcohol Starting Materials 1. methanol 2. ethanol 3. 1-propanol 4. 2-propanol 5. cyclohexanol Reagents available a. LIAIH4 f. PB13 k. CH3 CH2CH, MgBr; then H3 o* b. Н2SO4 g. CrОз, Н,SO4, H,О 1. Cg H5 MgBr (phenylmagnesium bromide); then H3 O+ с. НCI h. NaH m. (CH3)2 CHMGB1: then H3 O+ d. HBr i. CH3MGB3; then H3O* n. Dess-Martin periodinane (DMP) e. SOCI2 j. CH3 CH2MgBr; then H3O+
Specify both the alcohol starting material and the reagents you would use in each step in a synthesis of the compound shown. If the synthesis requires only two steps enter "none" for step 3. CI Alcohol Starting Materials 1. methanol 2. ethanol 3. 1-propanol 4. 2-propanol 5. cyclohexanol Reagents available a. LIAIH4 f. PB13 k. CH3 CH2CH, MgBr; then H3 o* b. Н2SO4 g. CrОз, Н,SO4, H,О 1. Cg H5 MgBr (phenylmagnesium bromide); then H3 O+ с. НCI h. NaH m. (CH3)2 CHMGB1: then H3 O+ d. HBr i. CH3MGB3; then H3O* n. Dess-Martin periodinane (DMP) e. SOCI2 j. CH3 CH2MgBr; then H3O+
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter16: Aldehydes And Ketones
Section: Chapter Questions
Problem 16.26P: Wittig reactions with the following -chloroethers can be used for the synthesis of aldehydes and...
Related questions
Question
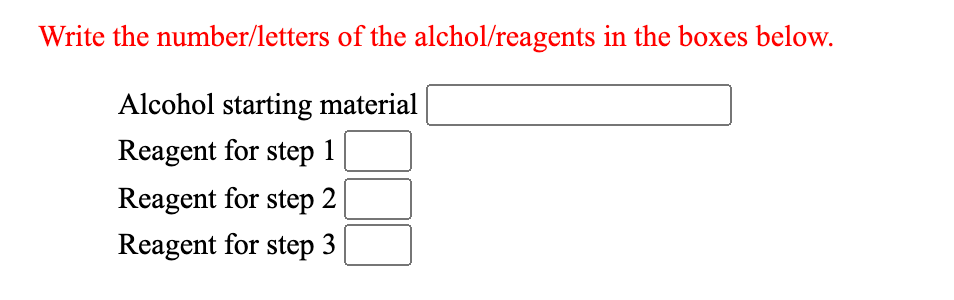
Transcribed Image Text:Write the number/letters of the alchol/reagents in the boxes below.
Alcohol starting material
Reagent for step 1
Reagent for step 2
Reagent for step 3
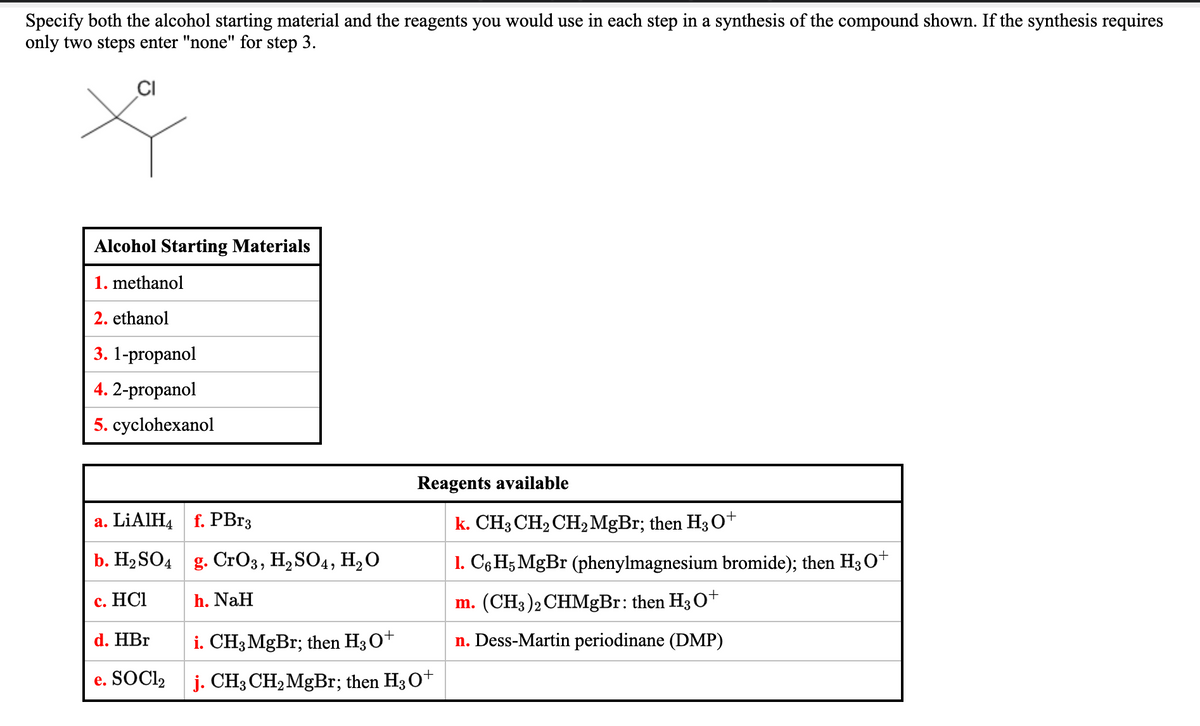
Transcribed Image Text:Specify both the alcohol starting material and the reagents you would use in each step in a synthesis of the compound shown. If the synthesis requires
only two steps enter "none" for step 3.
Alcohol Starting Materials
1. methanol
2. ethanol
3. 1-propanol
4. 2-propanol
5. cyclohexanol
Reagents available
а. LIAIHA f. PBIз
k. CH3 CH2 CH2 MGB3; then H3 O+
b. Н2SO4 g. CrОз, Н,SO4, H,О
1. C6H; MgBr (phenylmagnesium bromide); then H3 O+
с. НСІ
h. NaH
m. (CH3)2 CHMgBr: then H3O+
d. HBr
i. CH3MgBr; then H3O+
n. Dess-Martin periodinane (DMP)
e. SOCI2 j. CH; CH2 MgBr; then H3O*
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning