SS a. For the following balanced chemical equation, calculate how many moles of products would be produced if 0.255 mol of the first reactant were to react completely. CO₂(g) + 4H2(g) → CH4 (g) + 2H₂O (1) mol CH4 mol H₂O b. For the following balanced chemical equation, calculate how many moles of products would be produced if 0.790 mol of the first reactant were to react completely. BaCl₂ (aq) + 2AgNO3(aq) → 2AgCl(s) + Ba(NO3)2 (aq) mol AgCI mol Ba(NO3)2 c. For the following balanced chemical equation, calculate how many moles of products would be produced if 0.815 mol of the first reactant were to react completely. C3H8 (9)+502(g) → 4H₂O(1) + 3CO₂(g) Submit Answer mol H₂O mol CO₂ Retry Entire Group 9 more group attempts remaining
SS a. For the following balanced chemical equation, calculate how many moles of products would be produced if 0.255 mol of the first reactant were to react completely. CO₂(g) + 4H2(g) → CH4 (g) + 2H₂O (1) mol CH4 mol H₂O b. For the following balanced chemical equation, calculate how many moles of products would be produced if 0.790 mol of the first reactant were to react completely. BaCl₂ (aq) + 2AgNO3(aq) → 2AgCl(s) + Ba(NO3)2 (aq) mol AgCI mol Ba(NO3)2 c. For the following balanced chemical equation, calculate how many moles of products would be produced if 0.815 mol of the first reactant were to react completely. C3H8 (9)+502(g) → 4H₂O(1) + 3CO₂(g) Submit Answer mol H₂O mol CO₂ Retry Entire Group 9 more group attempts remaining
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.70PAE: 4.70 The particulate scale drawing shown depicts the products of a reaction between H2 and O2...
Related questions
Question
Transcribed Image Text:SS
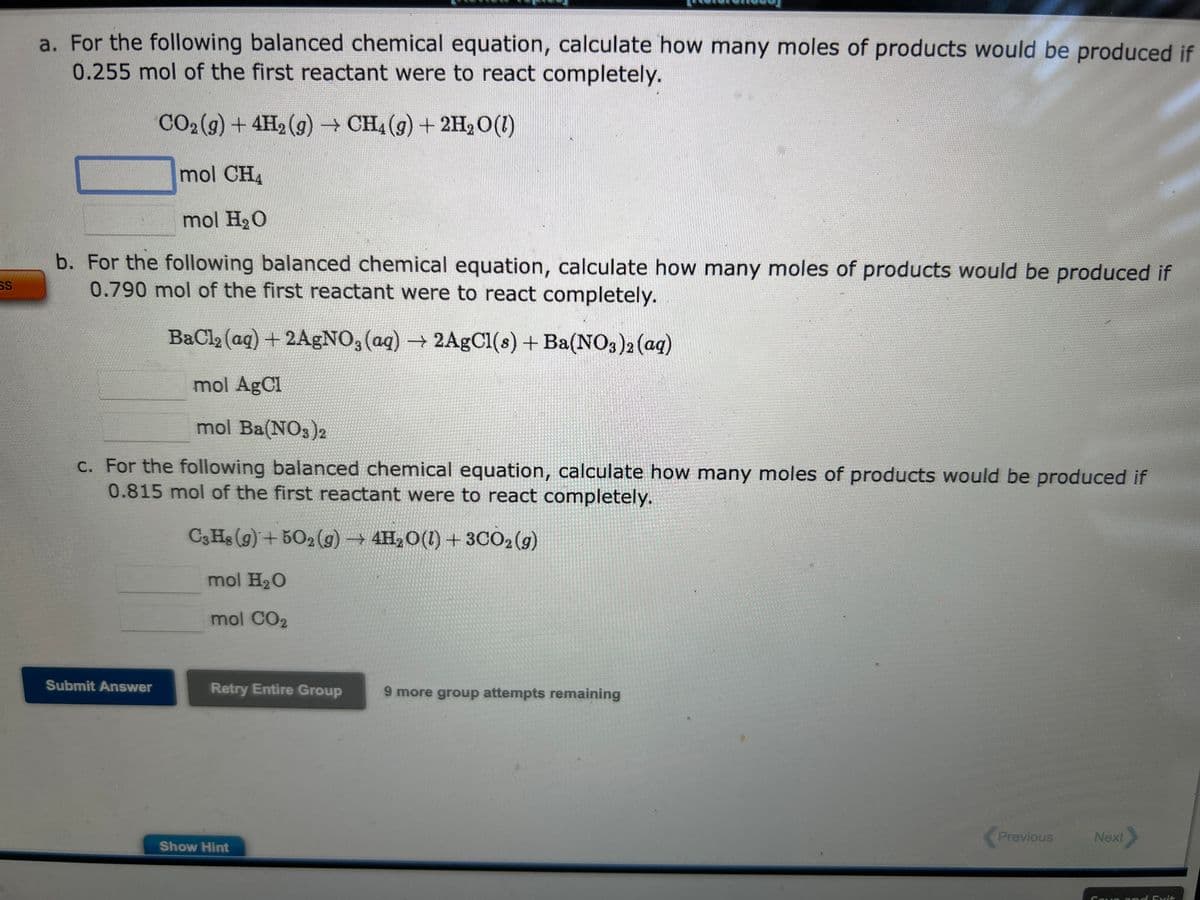
a. For the following balanced chemical equation, calculate how many moles of products would be produced if
0.255 mol of the first reactant were to react completely.
CO₂(g) + 4H₂(g) → CH4 (g) + 2H₂O (1)
mol CH₂
mol H₂O
b. For the following balanced chemical equation, calculate how many moles of products would be produced if
0.790 mol of the first reactant were to react completely.
BaCl₂ (aq) + 2AgNO3(aq) → 2AgCl(s) + Ba(NO3)2 (aq)
mol AgCl
mol Ba(NO3)2
c. For the following balanced chemical equation, calculate how many moles of products would be produced if
0.815 mol of the first reactant were to react completely.
C3H8 (9)+502(g) → 4H₂O(1) + 3CO2(g)
mol H₂O
mol CO2
Submit Answer
Retry Entire Group
Show Hint
9 more group attempts remaining
19
Previous
Next
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
