Standardisation of EDTA using zinc metal Mass of zinc = 1.050 g. Calculate the number of moles of EDTA in the average titre (show workings) Calculate the concentration of the EDTA solution (show workings) Calculate the concentration of the DILUTED EDTA solution (show workings) Sample No. 1 2 3 4 Final Burette reading (mL) 20.25 42.75 22.90 44.50 Initial Burette reading (mL) 0.05 20.25 1.50 22.90 Final-Initial Titre (mL) 20.20 22.50 21.4
Standardisation of EDTA using zinc metal Mass of zinc = 1.050 g. Calculate the number of moles of EDTA in the average titre (show workings) Calculate the concentration of the EDTA solution (show workings) Calculate the concentration of the DILUTED EDTA solution (show workings) Sample No. 1 2 3 4 Final Burette reading (mL) 20.25 42.75 22.90 44.50 Initial Burette reading (mL) 0.05 20.25 1.50 22.90 Final-Initial Titre (mL) 20.20 22.50 21.4
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter33: Automated Methods Of Analysis
Section: Chapter Questions
Problem 33.8QAP
Related questions
Question
please answer what you can. thank you so much in advance. :)
Standardisation of EDTA using zinc metal
Mass of zinc = 1.050 g.
- Calculate the number of moles of EDTA in the average titre (show workings)
- Calculate the concentration of the EDTA solution (show workings)
- Calculate the concentration of the DILUTED EDTA solution (show workings)
|
Sample No. |
1 |
2 |
3 |
4 |
|
Final Burette reading (mL)
|
20.25 |
42.75 |
22.90 |
44.50 |
|
Initial Burette reading (mL) |
0.05 |
20.25 |
1.50 |
22.90 |
|
Final-Initial Titre (mL) |
20.20 |
22.50 |
21.4 |
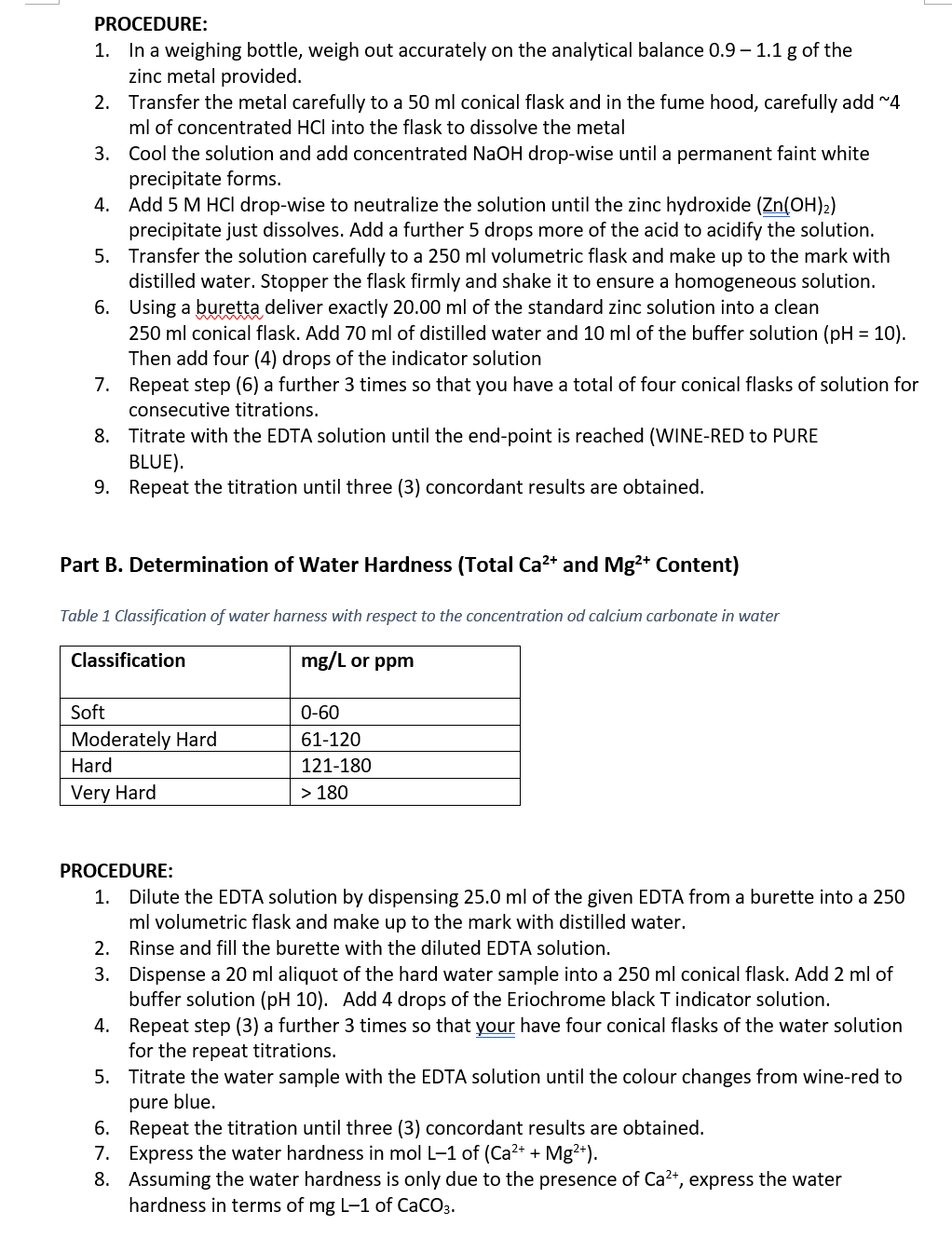
Transcribed Image Text:PROCEDURE:
1. In a weighing bottle, weigh out accurately on the analytical balance 0.9 - 1.1 g of the
zinc metal provided.
2.
Transfer the metal carefully to a 50 ml conical flask and in the fume hood, carefully add ~4
ml of concentrated HCI into the flask to dissolve the metal
3.
Cool the solution and add concentrated NaOH drop-wise until a permanent faint white
precipitate forms.
4. Add 5 M HCl drop-wise to neutralize the solution until the zinc hydroxide (Zn(OH)₂)
precipitate just dissolves. Add a further 5 drops more of the acid to acidify the solution.
Transfer the solution carefully to a 250 ml volumetric flask and make up to the mark with
distilled water. Stopper the flask firmly and shake it to ensure a homogeneous solution.
Using a buretta deliver exactly 20.00 ml of the standard zinc solution into a clean
5.
6.
250 ml conical flask. Add 70 ml of distilled water and 10 ml of the buffer solution (pH = 10).
Then add four (4) drops of the indicator solution
Repeat step (6) a further 3 times so that you have a total of four conical flasks of solution for
consecutive titrations.
7.
8.
Titrate with the EDTA solution until the end-point is reached (WINE-RED to PURE
BLUE).
9.
Repeat the titration until three (3) concordant results are obtained.
Part B. Determination of Water Hardness (Total Ca²+ and Mg²+ Content)
Table 1 Classification of water harness with respect to the concentration od calcium carbonate in water
mg/L or ppm
Classification
Soft
Moderately Hard
Hard
Very Hard
0-60
61-120
121-180
> 180
PROCEDURE:
1. Dilute the EDTA solution by dispensing 25.0 ml of the given EDTA from a burette into a 250
ml volumetric flask and make up to the mark with distilled water.
2. Rinse and fill the burette with the diluted EDTA solution.
3. Dispense a 20 ml aliquot of the hard water sample into a 250 ml conical flask. Add 2 ml of
buffer solution (pH 10). Add 4 drops of the Eriochrome black T indicator solution.
4.
Repeat step (3) a further 3 times so that your have four conical flasks of the water solution
for the repeat titrations.
5. Titrate the water sample with the EDTA solution until the colour changes from wine-red to
pure blue.
6. Repeat the titration until three (3) concordant results are obtained.
7. Express the water hardness in mol L-1 of (Ca²+ + Mg²+).
8. Assuming the water hardness is only due to the presence of Ca²+, express the water
hardness in terms of mg L-1 of CaCO3.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning