STARTING AMOUNT X Determine the total volume in milliliters of water a chemist should add if they want to prepare an 0.200 M aqueous solution with 30.2 g of NaCl. Assume the density of the resulting solution is the same as the water. ADD FACTOR x( ) 0.517 0.001 151 1.51 x 105 g NaCl/mol 0.200 g NaCl Question 5 of 17 1 6.022 x 1023 mL 22.99 L ANSWER 2.58 1000 2580 30.2 M NaCl RESET J 58.44 49.46 mol NaCl Submit +
STARTING AMOUNT X Determine the total volume in milliliters of water a chemist should add if they want to prepare an 0.200 M aqueous solution with 30.2 g of NaCl. Assume the density of the resulting solution is the same as the water. ADD FACTOR x( ) 0.517 0.001 151 1.51 x 105 g NaCl/mol 0.200 g NaCl Question 5 of 17 1 6.022 x 1023 mL 22.99 L ANSWER 2.58 1000 2580 30.2 M NaCl RESET J 58.44 49.46 mol NaCl Submit +
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter1: Introduction To Chemistry
Section: Chapter Questions
Problem 1.87PAE: 1.87 A solution of ethanol in water has a volume of 54.2 mL and a mass of 49.6 g. what information...
Related questions
Question
please help and put it in the correct form i keep doing it and its wrong.
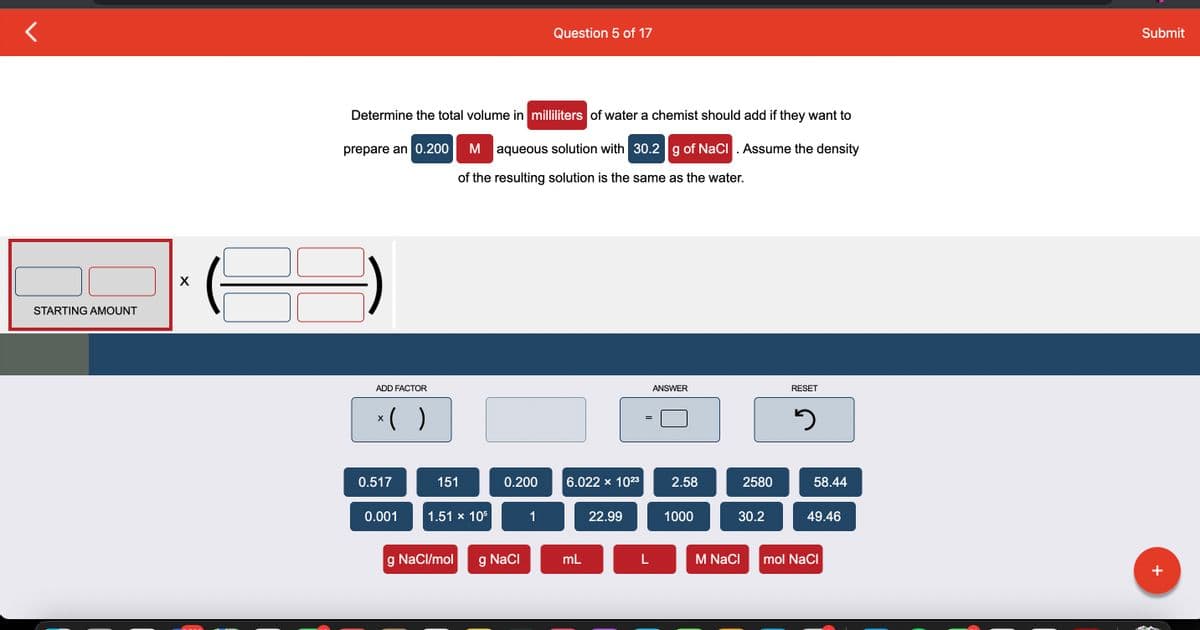
Transcribed Image Text:STARTING AMOUNT
X
Determine the total volume in milliliters of water a chemist should add if they want to
prepare an 0.200
M aqueous solution with 30.2 g of NaCl. Assume the density
of the resulting solution is the same as the water.
ADD FACTOR
x( )
X
0.517
0.001
151
1.51 x 105
g NaCl/mol
0.200
NaCl
Question 5 of 17
1
6.022 × 10²3
mL
22.99
L
ANSWER
2.58
1000
2580
30.2
M NaCl
RESET
3
58.44
49.46
mol NaCl
Submit
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning