Steam at a temperature of 133 'C is vented into an aluminum cup of mass 200 grams containing 576 grams of water both at 32.1 'C. The final temperature of the water (original + condensed) and the aluminum cup is 67.7 'C. C =1 cal/g-C, Csteam = 0.48 cal/g-C, CAI = 0.215 cal/g-C, Lsteam = 539 cal/g What is the mass of the steam condensed in grams?
Steam at a temperature of 133 'C is vented into an aluminum cup of mass 200 grams containing 576 grams of water both at 32.1 'C. The final temperature of the water (original + condensed) and the aluminum cup is 67.7 'C. C =1 cal/g-C, Csteam = 0.48 cal/g-C, CAI = 0.215 cal/g-C, Lsteam = 539 cal/g What is the mass of the steam condensed in grams?
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter17: Energy In Thermal Processes: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 2OQ: A 100-g piece of copper, initially at 95.0C, is dropped into 200 g of water contained in a 280-g...
Related questions
Question
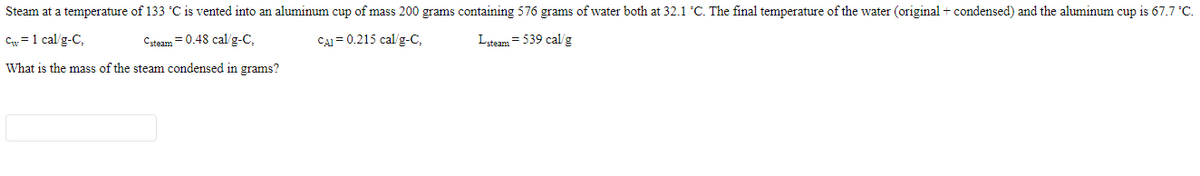
Transcribed Image Text:Steam at a temperature of 133 °C is vented into an aluminum cup of mass 200 grams containing 576 grams of water both at 32.1 °C. The final temperature of the water (original + condensed) and the aluminum cup is 67.7 °C.
Cw =1 cal/g-C,
Csteam = 0.48 cal/g-C,
CAI = 0.215 cal/g-C,
Lsteam = 539 cal/g
What is the mass of the steam condensed in grams?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning