Steam reforming of methane ( CH, ) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. An industrial chemist studying this reaction fills a 25.0 L tank with 1.8 mol of methane gas and 6.0 mol of water vapor, and when the mixture has come to equilibrium measures the amount of carbon monoxide gas to be 0.18 mol. Calculate the concentration equilibrium constant for the steam reforming of methane at the final temperature of the mixture. Round your answer to 2 significant digits. K = 0 Explanation Check O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center| Acces tv 23 MacBook Air DII DD 888 F7 FB F5 F6 F3 F4 F1 F2 & @ $ 7 8 1 2 4. 回 国 5
Steam reforming of methane ( CH, ) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. An industrial chemist studying this reaction fills a 25.0 L tank with 1.8 mol of methane gas and 6.0 mol of water vapor, and when the mixture has come to equilibrium measures the amount of carbon monoxide gas to be 0.18 mol. Calculate the concentration equilibrium constant for the steam reforming of methane at the final temperature of the mixture. Round your answer to 2 significant digits. K = 0 Explanation Check O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center| Acces tv 23 MacBook Air DII DD 888 F7 FB F5 F6 F3 F4 F1 F2 & @ $ 7 8 1 2 4. 回 国 5
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.101PAE: 12.101 An engineer working on a design to extract petroleum from a deep thermal reservoir wishes to...
Related questions
Question
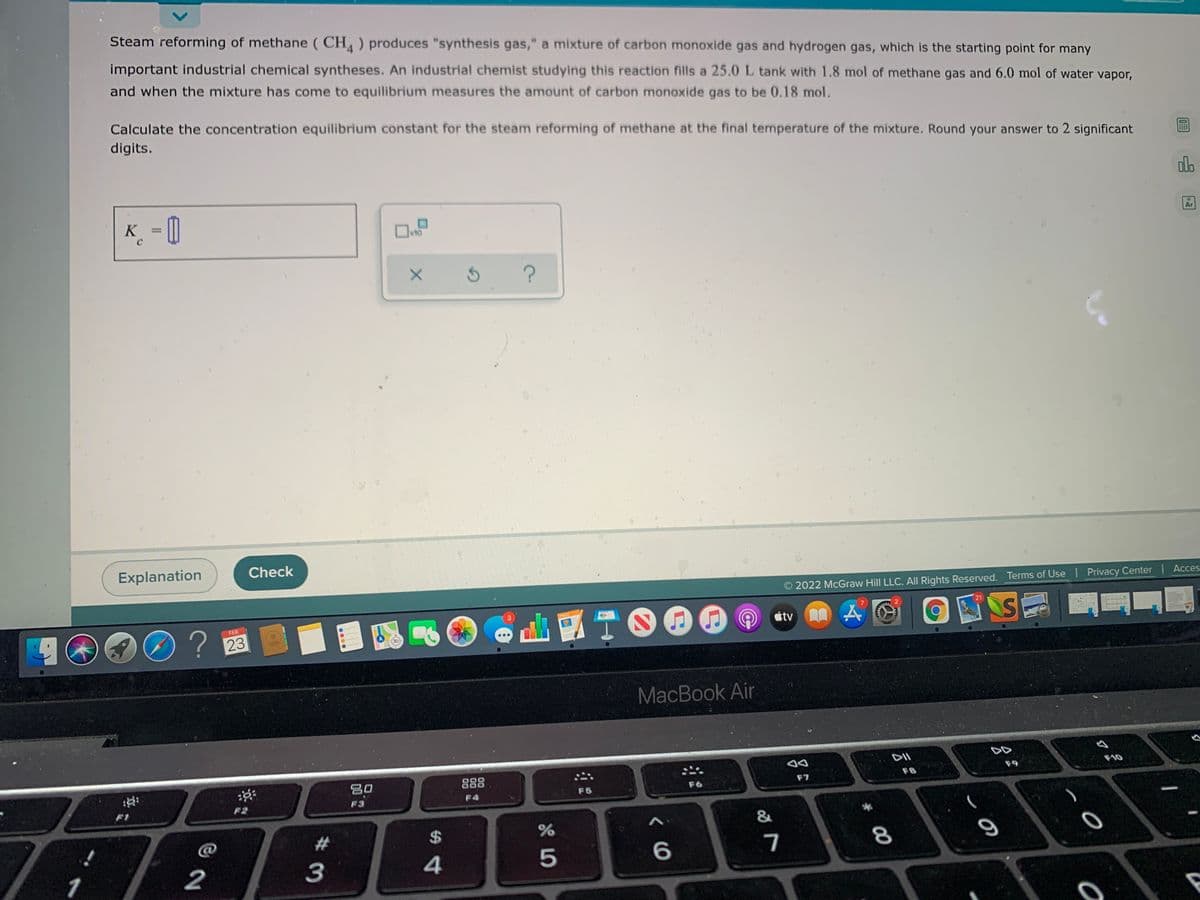
Transcribed Image Text:Steam reforming of methane ( CH ) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many
important industrial chemical syntheses. An industrial chemist studying this reaction fills a 25.0 L tank with 1.8 mol of methane gas and 6.0 mol of water vapor,
and when the mixture has come to equilibrium measures the amount of carbon monoxide gas to be 0.18 mol.
Calculate the concentration equilibrium constant for the steam reforming of methane at the final temperature of the mixture. Round your answer to 2 significant
digits.
olo
K_ = 0
=D=
%3D
x10
Ar
Explanation
Check
2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Acces.
TO00
2.
21
SE
FEB
tv
23
30
MacBook Air
DII
DD
80
888
F9
F10
F7
F8
F5
F6
F2
F3
F4
&
2#
%2$
4.
5
7
8
1
2
3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning