structure, the condensed structure, the skeletal structure, or the molecule's name. Use the given information to fill-in the other three items in the row. For the cyclic compound (fourth row), draw a side-view instead of a skeletal structure. A-C IH H 1 4 LINE BOND STRUCTURE TIL H HHHHH H-C-C=C-C- HL A CICIG-H -c=c-c² H HH H- H H-C H H 4101 HHH I H H. 1 с CIH H 1 C H H -H H H HH H H !=! H с 7 1 1 T 1 H H H H H Zcc-H H H I I C H !. H CONDENSED STRUCTURE CH3 1 CH₂=CH-CH₂-Cit-CH₂ - CH₂ CH3-CEC-CH₂-CH3 с H₂ CH₂ H /H 3 CH₂CH C=C CH₂ H -CH3 -4 SKELETAL STRUCTURE I have added dots to indicate the position of carbon atoms Draw the side-view CH₂CH3 CH3-CH₂-CH-CH-CH₂-CH₂-CH₂-CH₂ CH₂-CH₂-CH₂ d NAME 2,4-dimethylhexane 2-pentyne trans-2-pentyne trans-1,2- dimethylcyclopentane 3-ethyl- 14-propyl- octane
structure, the condensed structure, the skeletal structure, or the molecule's name. Use the given information to fill-in the other three items in the row. For the cyclic compound (fourth row), draw a side-view instead of a skeletal structure. A-C IH H 1 4 LINE BOND STRUCTURE TIL H HHHHH H-C-C=C-C- HL A CICIG-H -c=c-c² H HH H- H H-C H H 4101 HHH I H H. 1 с CIH H 1 C H H -H H H HH H H !=! H с 7 1 1 T 1 H H H H H Zcc-H H H I I C H !. H CONDENSED STRUCTURE CH3 1 CH₂=CH-CH₂-Cit-CH₂ - CH₂ CH3-CEC-CH₂-CH3 с H₂ CH₂ H /H 3 CH₂CH C=C CH₂ H -CH3 -4 SKELETAL STRUCTURE I have added dots to indicate the position of carbon atoms Draw the side-view CH₂CH3 CH3-CH₂-CH-CH-CH₂-CH₂-CH₂-CH₂ CH₂-CH₂-CH₂ d NAME 2,4-dimethylhexane 2-pentyne trans-2-pentyne trans-1,2- dimethylcyclopentane 3-ethyl- 14-propyl- octane
Chapter22: Organic And Biological Molecules
Section: Chapter Questions
Problem 6RQ: Distinguish between isomerism and resonance. Distinguish between structural and geometric isomerism....
Related questions
Question
I would like to you help me with my homework because I struggled
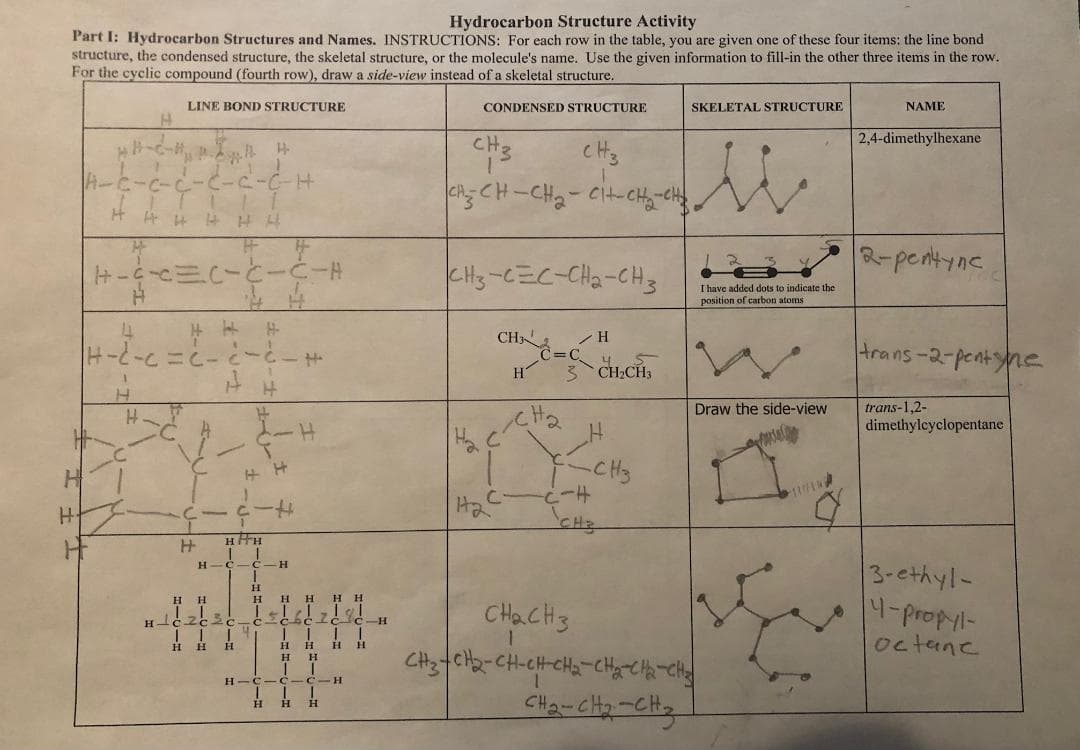
Transcribed Image Text:Hydrocarbon Structure Activity
Part I: Hydrocarbon Structures and Names. INSTRUCTIONS: For each row in the table, you are given one of these four items: the line bond
structure, the condensed structure, the skeletal structure, or the molecule's name. Use the given information to fill-in the other three items in the row.
For the cyclic compound (fourth row), draw a side-view instead of a skeletal structure.
LINE BOND STRUCTURE
H
H
H
E
C-C-C-C-C-H
1
M
H-C-M
1
44 14+ H4
H
11
|H-2-C=C-C
H
C-
H
HH H-
H
H
H H
I
H
-C-C-H
H.
1
30
1
H
1
H
T
1 4
↓
H
HH
C
HHH
1
I
C-H
- H
H
#
H H H HH
T
H
-Tel
Mcbc
1
H
H
1
H
H H
T
HIC-C-C-H
IC C-H
H
CONDENSED STRUCTURE
CH₂=CH-CH₂-Cit-CH₂ - CH₂
H₂
CH3-CEC-CH₂-CH3
1₂
CH3
CH3
H
CH₂
-C-H
H
CH₂CH3
CH3
SKELETAL STRUCTURE
w
I have added dots to indicate the
position of carbon atoms
Draw the side-view
1
Ở
CH₂CH3
CH3-CH₂-CH-CH-CH₂-CH₂-CH₂-CH₂
CH₂-CH₂-CH₂
NAME
2,4-dimethylhexane
2-pentyne
trans-2-pentyne
trans-1,2-
dimethylcyclopentane
3-ethyl-
14-propyl-
octane
Expert Solution
Step by step
Solved in 10 steps with 30 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning