Substance Mass (g) MW (g/mol) Mmol Equivalents OW: Sulfanilic acid 2-Naphthol NaNO2 Acid Orange 0.017 0.014 0.006 173.2 144.16 68.99 350, 32 0.1 0.1 0.1
Substance Mass (g) MW (g/mol) Mmol Equivalents OW: Sulfanilic acid 2-Naphthol NaNO2 Acid Orange 0.017 0.014 0.006 173.2 144.16 68.99 350, 32 0.1 0.1 0.1
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Please fill in the table
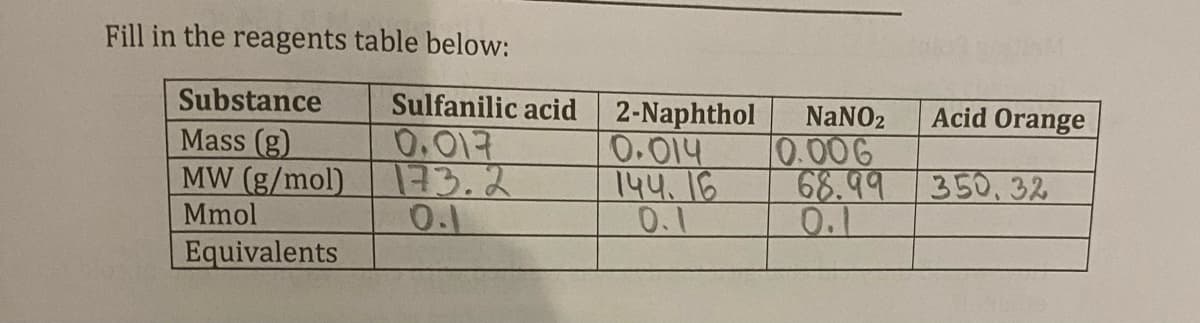
Transcribed Image Text:Fill in the reagents table below:
Substance
Sulfanilic acid
2-Naphthol NaNO2
Acid Orange
Mass (g)
0.017
0.014
0.006
MW (g/mol)
Mmol
173.2
144.16
68.99 350, 32
0.1
0.1
0.1
Equivalents

Transcribed Image Text:To a dry mortar and pestle add the three starting materials and gently mix the
solids. Do not pound dry nitrate salts as they pose a danger of explosion. Rather, use
a circular motion to grind the materials. Add a very small drop of water using a glass
pipette and then grind the materials slowly using circular motions to pulverize the
crystals for 10 min. Note any observations. You should obtain a wet powder. If you
obtained a paste, you have added too much water and would have to let the product
dry in air before proceeding.
Add ethyl acetate (5 mL) to the dry powder and continue to grind to dissolve any
unreacted starting material. Assemble a vacuum filtration with a Hirsh funnel
wetted with ethyl acetate. When you are ready, turn the vacuum on and transfer the
reaction mixture using a glass pipette to the center of the filter paper. Use additional
ethyl acetate (3 mL) to transfer the entirety of the product from the mortar, as well
as to wash the product. Discard the filtrate in the organic waste bottle and let the
precipitate air-dry for 15 min. The product is water soluble and somewhat
hygroscopic, avoid its exposure to humidity.
Weigh the solids to obtain the percent yield and determine its melting point. Note
that although the product was purified of the organic starting materials by the ethyl
acetate wash, there may be unreacted sodium nitrite left. For the purposes of this
lab, we will assume that all of the nitrite reacted. However, to fully purify the
product, the dye may be washed with saturated sodium chloride solution followed
by cold methanol.
Characterization
Obtain a UV-Vis spectrum of your product by dissolving 10 mg Acid Orange in 100
mL distilled water using a volumetric flask. Calculate the molarity of this solution.
Next, take 10 mL of this solution and dilute it with 100 mL of water using a
volumetric flask. Transfer the solutions in labeled beakers so others may use the
volumetric flask. Use M1V1= M2V2 to calculate the concentration of the second
solution.
Follow instructions on how to run the UV-Vis spectrometer. Briefly, run a blank
sample in water. Place your sample in a cuvette using a pipette. The reading on the
y-axis must be between 0.2 - 1.0 absorption units, otherwise your sample is too
dilute or concentrated. Note the maxima of the peaks observed, both in wavelength
and absorption units (A). Calculate the molar absorptivity at the maximum
wavelength (max) by using Beer's Law equation, & = A*b*c
where & is the molar absoprtivity, A is the absortion, and c is the molar
concentration. Of course, more than one concentration must be measured to get an
accurate result. For the purposes of this lab, a single measurement will suffice. Print
your spectrum and this attach to your laboratory report.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY