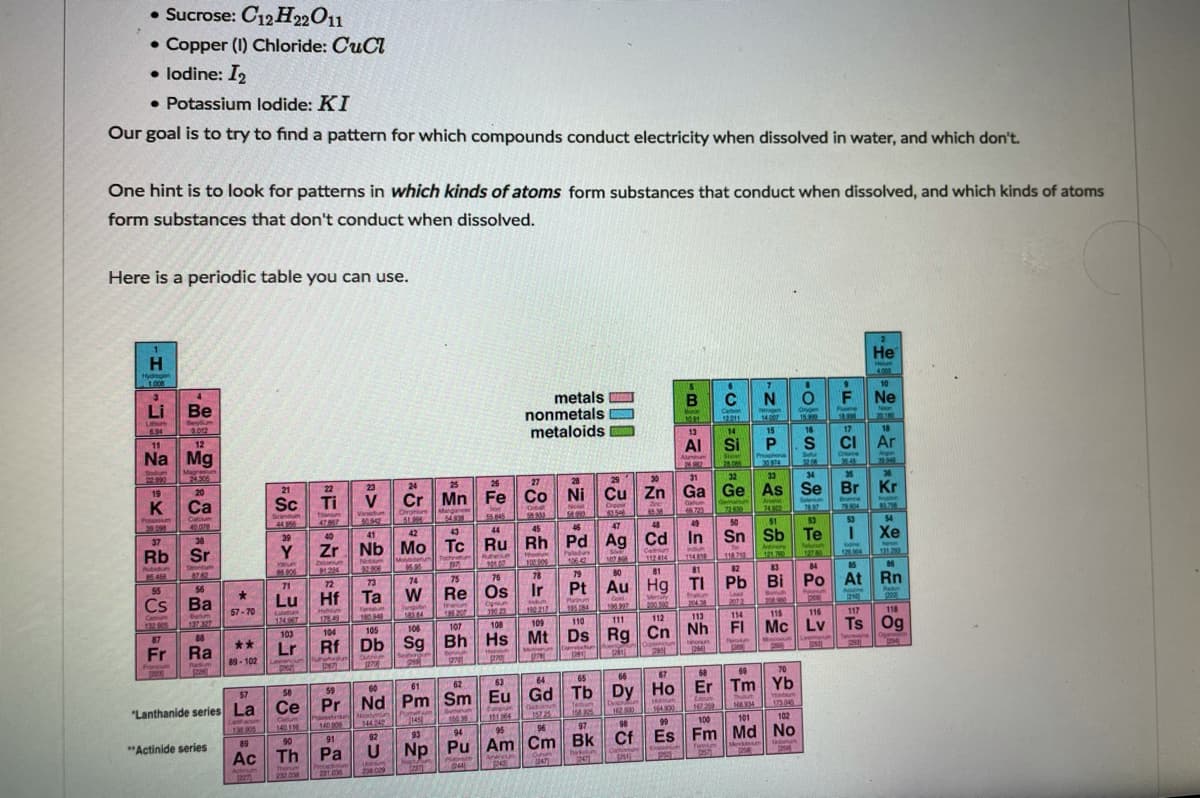
• Sucrose: C12 H22O11 • Copper (I) Chloride: CuCl • lodine: I2 • Potassium lodide: KI Our goal is to try to find a pattern for which compounds conduct electricity when dissolved in water, and which don't. One hint is to look for patterns in which kinds of atoms form substances that conduct when dissolved, and which kinds of atoms form substances that don't conduct when dissolved.
• Sucrose: C12 H22O11 • Copper (I) Chloride: CuCl • lodine: I2 • Potassium lodide: KI Our goal is to try to find a pattern for which compounds conduct electricity when dissolved in water, and which don't. One hint is to look for patterns in which kinds of atoms form substances that conduct when dissolved, and which kinds of atoms form substances that don't conduct when dissolved.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter2: Atoms, Molecules, And Ions
Section: Chapter Questions
Problem 2.118QE
Related questions
Question
Transcribed Image Text:• Sucrose: C12 H22O11
• Copper (I) Chloride: CuCl
• lodine: I2
• Potassium lodide: KI
Our goal is to try to find a pattern for which compounds conduct electricity when dissolved in water, and which don't.
One hint is to look for patterns in which kinds of atoms form substances that conduct when dissolved, and which kinds of atoms
form substances that don't conduct when dissolved.
Here is a periodic table you can use.
H.
Не
yrgen
1008
Helum
4000
10
metals
nonmetals
metaloids
Li
Be
Ne
Carbon
Ovgn
Pucene
Neon
Lihum
6.54
Berylum
9012
11
12
13
14
15
16
17
18
Na Mg
Al
Si
P.
CI
Ar
Prosphonue
00.974
Sutu
Cre
35.45
Argn
Sodium
22 999
Magresiu
4305
28.982
32.06
310
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
19
20
K
Ca
Sc
Ti
V
Cr Mn Fe Co
Ni Cu
Zn Ga
Ge As
Se Br
Kr
Galum
6.723
Bomre
Cebat
Cnppr
Scandum
44956
Oron
51
Tnum
Vanadum
72630
79.904
Calcium
40.07
55.845
N
2492
20.0
45
46
47
48
49
50
51
53
53
54
37
38
39
40
41
42
43
44
Rb Sr
Zr Nb Mo Tc Ru Rh Pd Ag
Cd In
Sn Sb Te
Xe
Telu
127A0
thodu
Paledur
Caum
Andineny
ndne
eon
Notum
Maoenum
114.88
11 710
131 293
Siantium
874
Pubidum
107
112414
9124
78
79
80
81
81
82
83
84
85
86
71
72
73
74
75
76
Po At
Rn
55
56
Lu
Hf
Ta
W
Re Os
Ir
Pt Au
Hg TI
Pb
Bi
Cs
Ba
Radn
Leed
2072
induh
Parinum
Coid
Merculy
57-70
2000
pee
Tanta
irenu
letur
124 967
Haum
Cesium
132.005
Berum
13727
8217
109
171.4
114
115
116
117
118
105
106
107
108
110
111
112
113
Mc Lv Ts Og
87
88
103
104
FI
Db Sg Bh Hs Mt
Ds Rg Cn Nh
Fr
Ra
**
Lr
Rf
hhoren
2501
293
254
Radum
89- 102
I47
$270
pe
65
66
67
68
69
70
61
62
63
64
Yb
Nd Pm Sm Eu Gd Tb Dy Ho
Curpu
60
Er Tm
$7
58
59
Ce Pr
Thulun
"Lanthanide series La
Lantaue
Loun
16729
Terbrum
164300
173040
Cotun
140.116
brnNoodymun
6
144249
140 90
Pumesum
145
142500
13.805
97
98
99
100
101
102
92
93
94
95
96
"Actinide series
89
90
91
U
Np Pu Am Cm Bk Cf Es Fm Md No
Ac Th
Pa
Formum
250
Amereum
47)
2471
Actinum
227
Thenum
230008
3227
201.006
Transcribed Image Text:3. Let's repeat this process for the atoms that make up the substances that do not conduct when dissolved in water. What do you
notice now?
BIU
E E E E
回fた
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning