Suppose an electron in a hydrogen atom is in a 2p state, and the radial wavefunction -e zao, where a, is the Bohr radius. (2a.)3/2 /3a, а) What possible angles might the angular momentum vector L make with the z-axis? b) What is the most probable radius (in terms of a.) at which the electron is found? c) What is the expectation value of r in this state? Note: r°e#dx = 120. %3D
Suppose an electron in a hydrogen atom is in a 2p state, and the radial wavefunction -e zao, where a, is the Bohr radius. (2a.)3/2 /3a, а) What possible angles might the angular momentum vector L make with the z-axis? b) What is the most probable radius (in terms of a.) at which the electron is found? c) What is the expectation value of r in this state? Note: r°e#dx = 120. %3D
Related questions
Question
How to solve this question
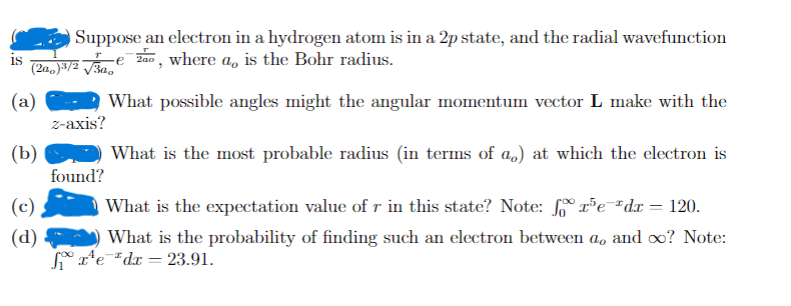
Transcribed Image Text:Suppose an electron in a hydrogen atom is in a 2p state, and the radial wavefunction
Za0, where a, is the Bohr radius.
is
(2a,)3/2 V3a,
(a)
What possible angles might the angular momentum vector L make with the
2-ахis?
(b)
What is the most probable radius (in terms of a,) at which the electron is
found?
(c)
What is the expectation value of r in this state? Note: rPe"dx = 120.
(d)
What is the probability of finding such an electron between a, and oo? Note:
* r'e*dx = 23.91.
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps
