Suppose some nitrogen gas is contained in a cylinder whose volume is 0.00830 m³. The pressure in the box is 3.44 X 106 Pascals, and the temperature is 39°C. (Molecular weight of = 28) Find (a) the number of moles of nitrogen in the gas in the box, (b) the mass of one molecule of nitrogen (N2) (b) the average speed of a molecule in the gas.
Suppose some nitrogen gas is contained in a cylinder whose volume is 0.00830 m³. The pressure in the box is 3.44 X 106 Pascals, and the temperature is 39°C. (Molecular weight of = 28) Find (a) the number of moles of nitrogen in the gas in the box, (b) the mass of one molecule of nitrogen (N2) (b) the average speed of a molecule in the gas.
Physics for Scientists and Engineers: Foundations and Connections
1st Edition
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Katz, Debora M.
Chapter19: Temperature, Thermal Expansion And Gas Laws
Section: Chapter Questions
Problem 61PQ
Related questions
Question

Transcribed Image Text:Tc = TK - 273 ° C
T. +32
5
%3D
AL = a L,AT
AA = y A,AT y= 2a
AV = BV,AT B = 3a
Thermal Expansion
aL,AT
P1V1 = P2V2
Ti
AL =
T2
AA =y A,AT y = 2a
1/2 mv2 = 3/2kBT
AV = BV,AT ß = 3a
where kB is Boltzmann’s constant,
PV =
„RT
KB = 1.38 x 10-23 J/k
number of moles, n =
mass (grams)
molecular weight
Avogadro's number, NA = 6.02 x 1023
R = 8.31 J/mole-K
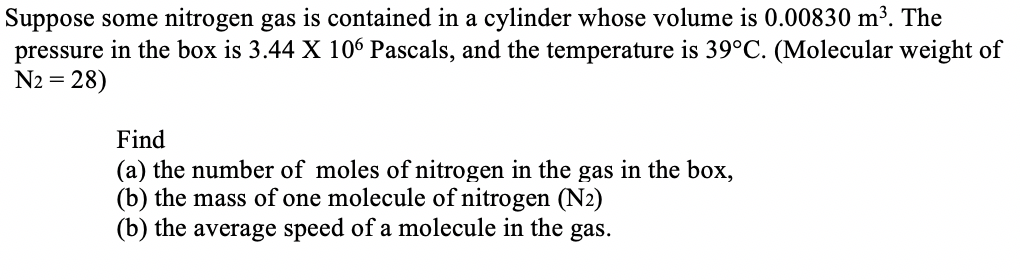
Transcribed Image Text:Suppose some nitrogen gas is contained in a cylinder whose volume is 0.00830 m³. The
pressure in the box is 3.44 X 106 Pascals, and the temperature is 39°C. (Molecular weight of
= 28)
Find
(a) the number of moles of nitrogen in the gas in the box,
(b) the mass of one molecule of nitrogen (N2)
(b) the average speed of a molecule in the gas.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning