Suppose that in a certain chemical process the reaction time y (hr) is related to the temperature (°F) in the chamber in which the reaction takes place according to the simple linear regression model with equation y = 4.10 - 0.01x and o = 0.07. A USE SALT (a) What is the expected change in reaction time for a 1°F increase in temperature? For a 9°F increase in temperature? 1°F increase hr 9°F increase hr (b) What is the expected reaction time when temperature is 190°F? When temperature is 260°F? 190°F hr 260°F hr (c) Suppose five observations are made independently on reaction time, each one for a temperature of 260°F. What is the probability that all five times are between 1.39 and 1.61 hours? (Round your answer to four decimal places.) (d) What is the probability that two independently observed reaction times for temperatures 1° apart are such that the time at the higher temperature exceeds the time at the lower temperature? (Round your answer to four decimal places.)
Suppose that in a certain chemical process the reaction time y (hr) is related to the temperature (°F) in the chamber in which the reaction takes place according to the simple linear regression model with equation y = 4.10 - 0.01x and o = 0.07. A USE SALT (a) What is the expected change in reaction time for a 1°F increase in temperature? For a 9°F increase in temperature? 1°F increase hr 9°F increase hr (b) What is the expected reaction time when temperature is 190°F? When temperature is 260°F? 190°F hr 260°F hr (c) Suppose five observations are made independently on reaction time, each one for a temperature of 260°F. What is the probability that all five times are between 1.39 and 1.61 hours? (Round your answer to four decimal places.) (d) What is the probability that two independently observed reaction times for temperatures 1° apart are such that the time at the higher temperature exceeds the time at the lower temperature? (Round your answer to four decimal places.)
College Algebra
7th Edition
ISBN:9781305115545
Author:James Stewart, Lothar Redlin, Saleem Watson
Publisher:James Stewart, Lothar Redlin, Saleem Watson
Chapter1: Equations And Graphs
Section: Chapter Questions
Problem 10T: Olympic Pole Vault The graph in Figure 7 indicates that in recent years the winning Olympic men’s...
Related questions
Question
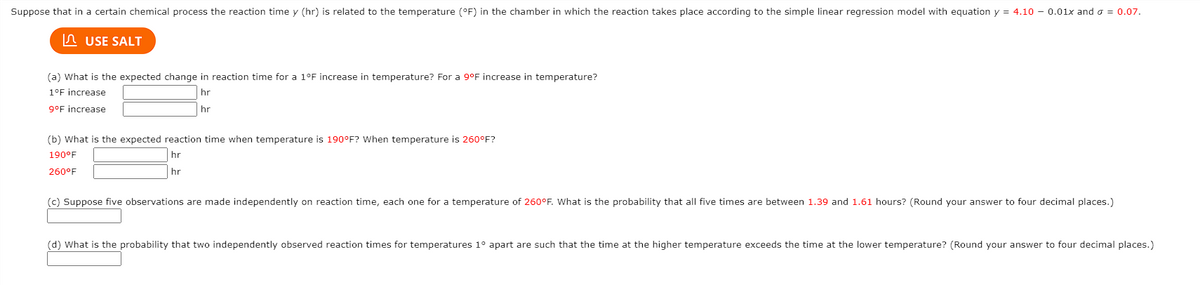
Transcribed Image Text:Suppose that in a certain chemical process the reaction time y (hr) is related to the temperature (°F) in the chamber in which the reaction takes place according to the simple linear regression model with equation y = 4.10 - 0.01x and o = 0.07.
n USE SALT
(a) What is the expected change in reaction time for a 1°F increase in temperature? For a 9°F increase in temperature?
1°F increase
hr
g°F increase
hr
(b) What is the expected reaction time when temperature is 190°F? When temperature is 260°F?
190°F
hr
260°F
hr
(c) Suppose five observations are made independently on reaction time, each one for a temperature of 260°F. What is the probability that all five times are between 1.39 and 1.61 hours? (Round your answer to four decimal places.)
(d) What is the probability that two independently observed reaction times for temperatures 1° apart are such that the time at the higher temperature exceeds the time at the lower temperature? (Round your answer to four decimal places.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

College Algebra
Algebra
ISBN:
9781305115545
Author:
James Stewart, Lothar Redlin, Saleem Watson
Publisher:
Cengage Learning

Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage

Algebra and Trigonometry (MindTap Course List)
Algebra
ISBN:
9781305071742
Author:
James Stewart, Lothar Redlin, Saleem Watson
Publisher:
Cengage Learning

College Algebra
Algebra
ISBN:
9781305115545
Author:
James Stewart, Lothar Redlin, Saleem Watson
Publisher:
Cengage Learning

Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage

Algebra and Trigonometry (MindTap Course List)
Algebra
ISBN:
9781305071742
Author:
James Stewart, Lothar Redlin, Saleem Watson
Publisher:
Cengage Learning

Trigonometry (MindTap Course List)
Trigonometry
ISBN:
9781305652224
Author:
Charles P. McKeague, Mark D. Turner
Publisher:
Cengage Learning
