Suppose that the microwave radiation has a wavelength of 12.4 cm. How many photons are required to heat 295 mL of coffee from 25.0 °C to 62.0 °C? Assume that the coffee has the same density, 0.997 g/mL, and specific heat capacity, 4.184 J/(g - K), as water over this temperature range. Express the number of photons numerically. View Available Hint(s) ΑΣφ photons
Suppose that the microwave radiation has a wavelength of 12.4 cm. How many photons are required to heat 295 mL of coffee from 25.0 °C to 62.0 °C? Assume that the coffee has the same density, 0.997 g/mL, and specific heat capacity, 4.184 J/(g - K), as water over this temperature range. Express the number of photons numerically. View Available Hint(s) ΑΣφ photons
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU3: Weather: Phase Changes And Behaviour Of Gases
SectionU3.18: Feeling Humid: Humidity, Condensation
Problem 9E
Related questions
Question
Transcribed Image Text:ecollege.com/course.html?courseld=16985674&OpenVellumHMAC=d4abff74a80d41d197d6c57220120253#10001
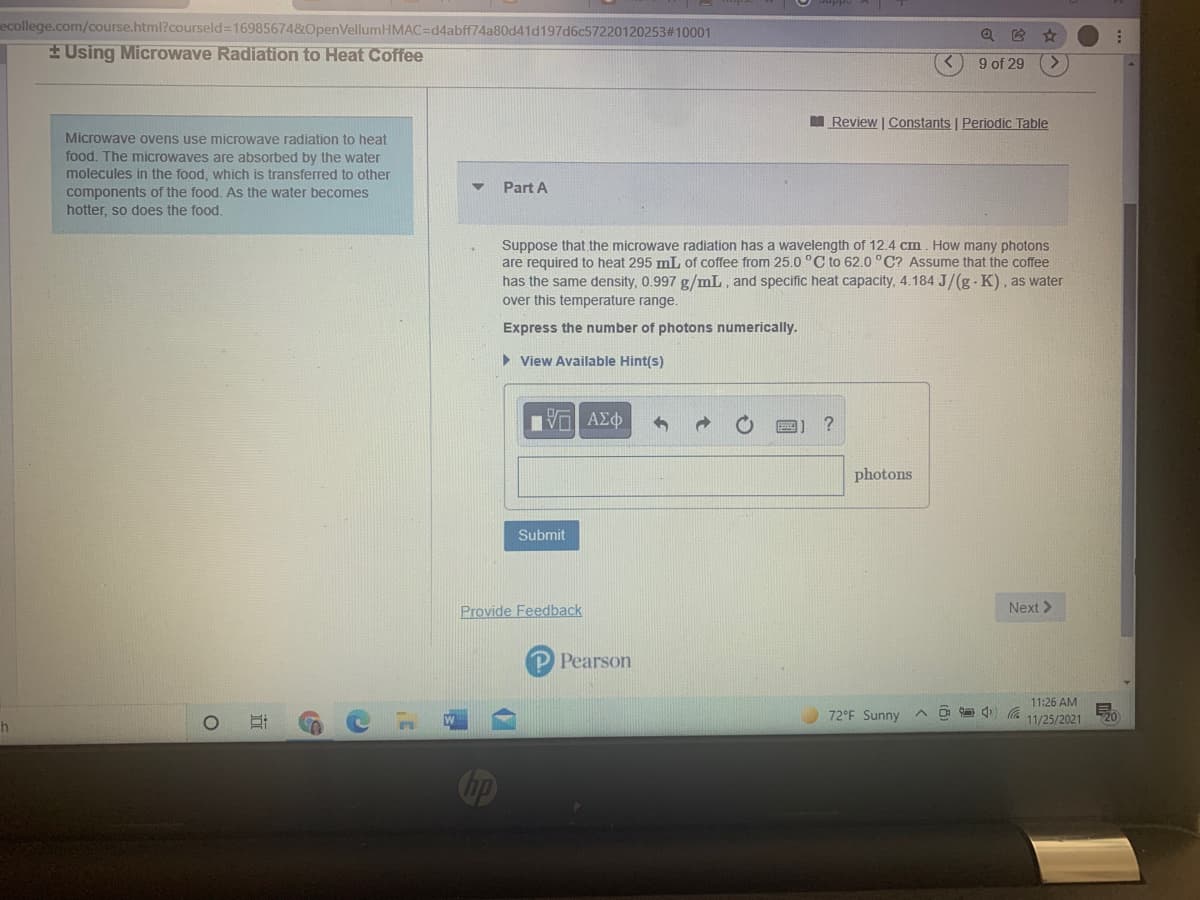
EUsing Microwave Radiation to Heat Coffee
9 of 29
M Review | Constants | Periodịc Table
Microwave ovens use microwaye radiation to heat
food. The microwaves are absorbed by the water
molecules in the food, which is transferred to other
components of the food. As the water becomes
hotter, so does the food.
Part A
Suppose that the microwave radiation has a wavelength of 12.4 cm. How many photons
are required to heat 295 mL of coffee from 25.0 °C to 62.0 °C? Assume that the coffee
has the same density, 0.997 g/mL, and specific heat capacity, 4.184 J/(g - K), as water
over this temperature range.
Express the number of photons numerically.
> View Available Hint(s)
?
photons
Submit
Provide Feedback
Next>
P Pearson
11:26 AM
72°F Sunny
11/25/2021
20
Cop
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER


Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER
