Suppose that you plan to make 10 grams of MgCO₃ by reacting MgSO4 with Na₂CO₃ [Figure 10]. However, Na₂CO₃ is unavailable and you need to replace it with K₂CO₃. Which statement is correct? *
Suppose that you plan to make 10 grams of MgCO₃ by reacting MgSO4 with Na₂CO₃ [Figure 10]. However, Na₂CO₃ is unavailable and you need to replace it with K₂CO₃. Which statement is correct? *
Chapter4: Calculations Used In Analytical Chemistry
Section: Chapter Questions
Problem 4.10QAP
Related questions
Question
Suppose that you plan to make 10 grams of MgCO₃ by reacting MgSO4 with Na₂CO₃ [Figure 10]. However, Na₂CO₃ is unavailable and you need to replace it with K₂CO₃. Which statement is correct? *
A - It can be predicted form the periodic table that the yield of MgCO₃ will be more when using K₂CO₃ because potassium is more reactive than sodium.
B - It can be predicted form the periodic table that the precipitate of MgCO₃ will form faster because potassium is more reactive than sodium.
C - None of these statements is correct.
D - Both these statements are correct.
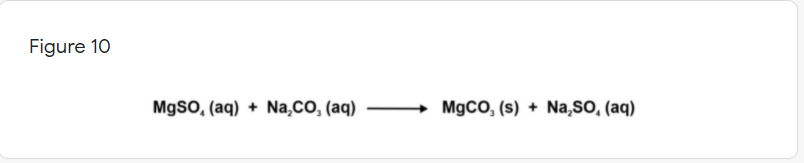
Transcribed Image Text:Figure 10
MgsO, (aq) + Na,CO, (aq)
MgCO, (s) + Na,SO, (aq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
