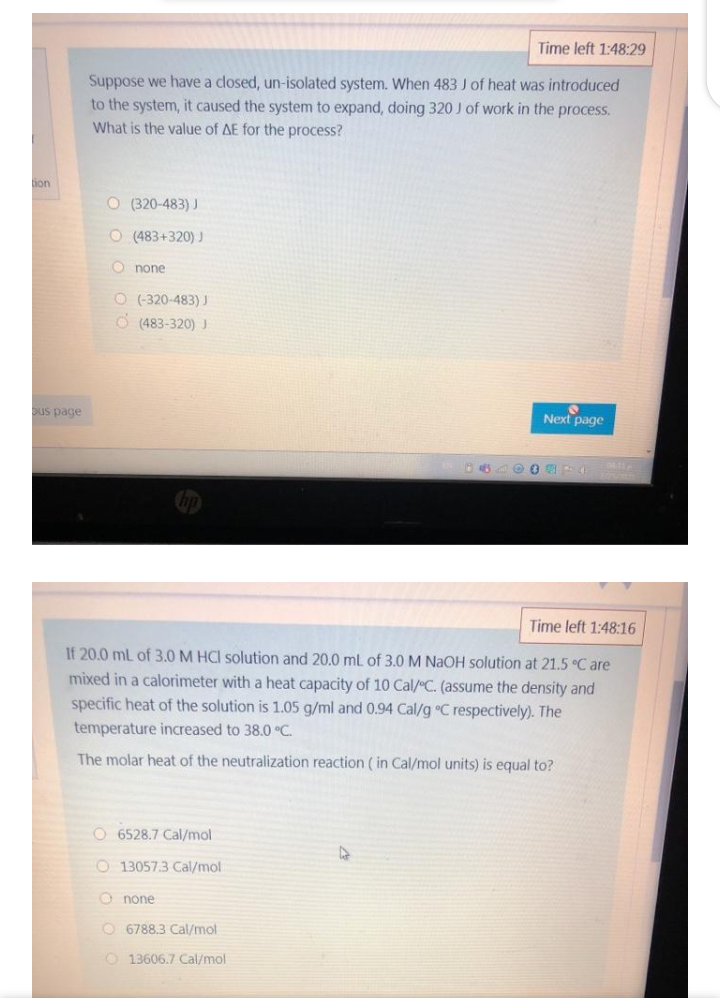
Suppose we have a closed, un-isolated system. When 483 J of heat was introduced to the system, it caused the system to expand, doing 320 J of work in the process. What is the value of AE for the process?
Suppose we have a closed, un-isolated system. When 483 J of heat was introduced to the system, it caused the system to expand, doing 320 J of work in the process. What is the value of AE for the process?
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter6: Thermochemistry
Section: Chapter Questions
Problem 66E: In a coffee-cup calorimeter, 100.0 mL of 1.0 M NaOH and 100.0 mL of 1.0 M HCI are mixed. Both...
Related questions
Question
100%
Transcribed Image Text:Time left 1:48:29
Suppose we have a closed, un-isolated system. When 483 J of heat was introduced
to the system, it caused the system to expand, doing 320 J of work in the process.
What is the value of AE for the process?
ion
O (320-483) J
O (483+320) J
O none
O (-320-483) J
O (483-320) J
pus page
Next page
Time left 1:48:16
If 20.0 mL of 3.0 M HCI solution and 20.0 mL of 3.0 M NaOH solution at 21.5 °C are
mixed in a calorimeter with a heat capacity of 10 Cal/ C. (assume the density and
specific heat of the solution is 1.05 g/ml and 0.94 Cal/g °C respectively). The
temperature increased to 38.0 °C.
The molar heat of the neutralization reaction ( in Cal/mol units) is equal to?
O 6528.7 Cal/mol
O 13057.3 Cal/mol
O none
O 6788.3 Cal/mol
O 13606.7 Cal/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning