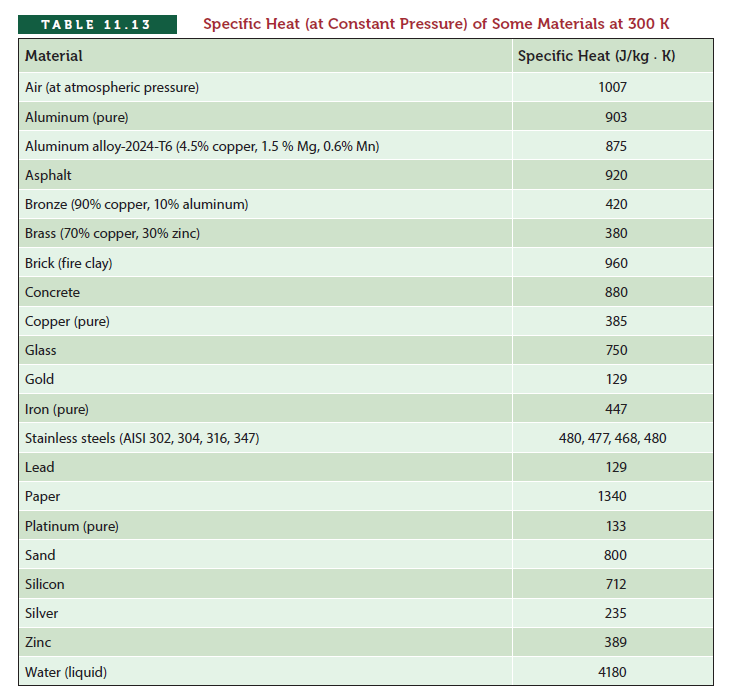
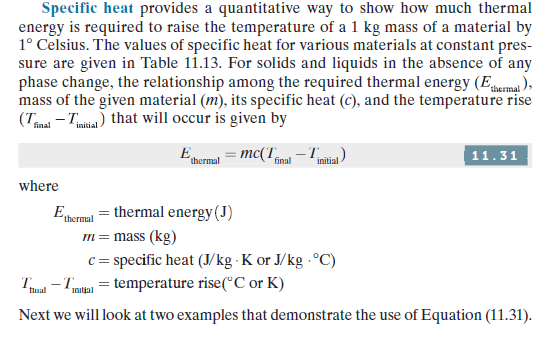
ТАBLE 11.13 Specific Heat (at Constant Pressure) of Some Materials at 300 K Material Specific Heat (J/kg . K) Air (at atmospheric pressure) 1007 Aluminum (pure) 903 Aluminum alloy-2024-T6 (4.5% copper, 1.5 % Mg, 0.6% Mn) 875 Asphalt 920 Bronze (90% copper, 10% aluminum) 420 Brass (70% copper, 30% zinc) 380 Brick (fire clay) 960 Concrete 880 Copper (pure) 385 Glass 750 Gold 129 Iron (pure) 447 Stainless steels (AISI 302, 304, 316, 347) 480, 477, 468, 480 Lead 129 Рaper 1340 Platinum (pure) 133 Sand 800 Silicon 712 Silver 235 Zinc 389 Water (liquid) 4180 Specific heat provides a quantitative way to show how much thermal energy is required to raise the temperature of a 1 kg mass of a material by 1° Celsius. The values of specific heat for various materials at constant pres- sure are given in Table 11.13. For solids and liquids in the absence of any phase change, the relationship among the required thermal energy (Eema ), mass of the given material (m), its specific heat (c), and the temperature rise (Tmal - Tmitia) that will occur is given by final E = mc(T -T 11.31 thermal final initial - where Eermul = thermal energy(J) m= mass (kg) c = specific heat (J/kg · K or J/kg - °C) T - Tmtan = temperature rise(°C or K) flual Initjal Next we will look at two examples that demonstrate the use of Equation (11.31).
We have exposed 1 kg of water, 1 kg of brick, and 1 kg of concrete each to a heat source that puts out 100 J every second. Assuming that all of the supplied energy goes to each material and they were all initially at the same temperature, which one of these materials will have a greater temperature rise after 10 s? We can answer this question using Equation as shown . We will first look up the values of the specific heat for water, brick, and concrete, which are cwater = 4180 J⁄kg K, cbrick = 960 J⁄kg K and cconcrete = 880 J⁄kg K . Now applying as shown , Ethermal = mc(Tfinal Tinitial) to each situation, it should be clear that although each material has the same amount of mass and is exposed to the same amount of thermal energy, the concrete will experience a higher temperature rise because it has the lowest heat capacity value among the three given materials.
Step by step
Solved in 2 steps


