Table 2: Molarity of H2O2 and KI and Reaction Rate Trial H2O2 Concentration, M KI Concentration, M Reaction Rate (Reciprocal Slope) 1 0.29 M 0.40 M 14.08 2 0.29 M 0.20M 25 3 0.023 M 0.40 M 20 Please help me with this part, and please show me all steps Order with respect to H2O2: Order with respect to KI:
Table 2: Molarity of H2O2 and KI and Reaction Rate Trial H2O2 Concentration, M KI Concentration, M Reaction Rate (Reciprocal Slope) 1 0.29 M 0.40 M 14.08 2 0.29 M 0.20M 25 3 0.023 M 0.40 M 20 Please help me with this part, and please show me all steps Order with respect to H2O2: Order with respect to KI:
Chapter5: Errors In Chemical Analyses
Section: Chapter Questions
Problem 5.10QAP
Related questions
Question
Table 2: Molarity of H2O2 and KI and Reaction Rate
|
Trial |
H2O2 Concentration, M |
KI Concentration, M |
Reaction Rate |
|
1 |
0.29 M |
0.40 M |
14.08 |
|
2 |
0.29 M |
0.20M |
25 |
|
3 |
0.023 M |
0.40 M |
20 |
Please help me with this part, and please show me all steps
- Order with respect to H2O2:
- Order with respect to KI:
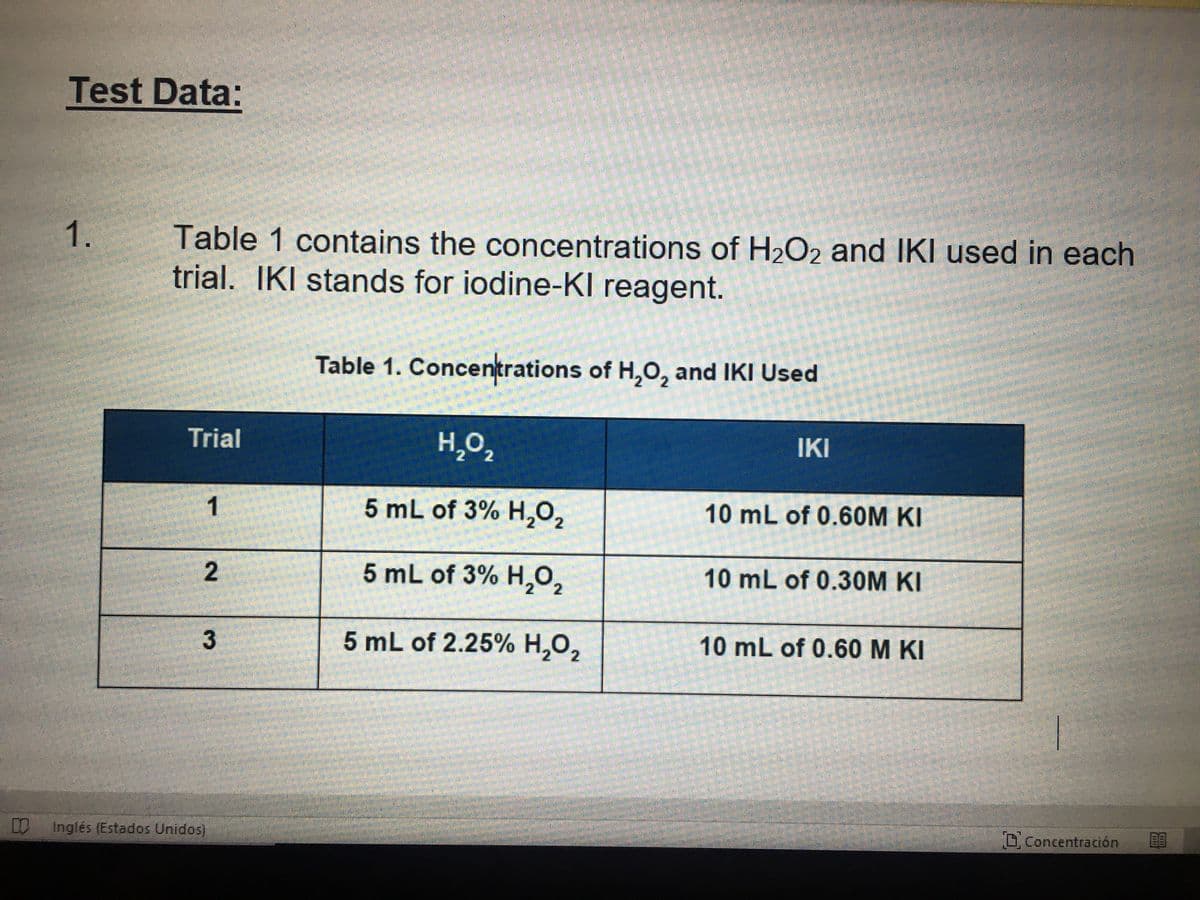
Transcribed Image Text:Test Data:
1.
Table 1 contains the concentrations of H2O2 and IKI used in each
trial. IKI stands for iodine-KI reagent.
Table 1. Concentrations of H,O, and IKI Used
Trial
H,O,
IKI
1
5 mL of 3% H,02
10 mL of 0.60M KI
5 mL of 3% H,0,
10 mL of 0.30M KI
5 mL of 2.25% H,0,
10 mL of 0.60 M KI
3.
O Inglés (Estados Unidos)
OConcentración
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

