Table salt, NaCl(s), and sugar, C₁₂H₂O₁1 (s), are accidentally mixed. A 5.50 g sample is burned, and 3.00 g of CO₂ (g) is produced. What was the mass percentage of the table salt in the mixture? percentage of NaCl: %
Table salt, NaCl(s), and sugar, C₁₂H₂O₁1 (s), are accidentally mixed. A 5.50 g sample is burned, and 3.00 g of CO₂ (g) is produced. What was the mass percentage of the table salt in the mixture? percentage of NaCl: %
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.87PAE
Related questions
Question
How can we find the percentage? And the produced?
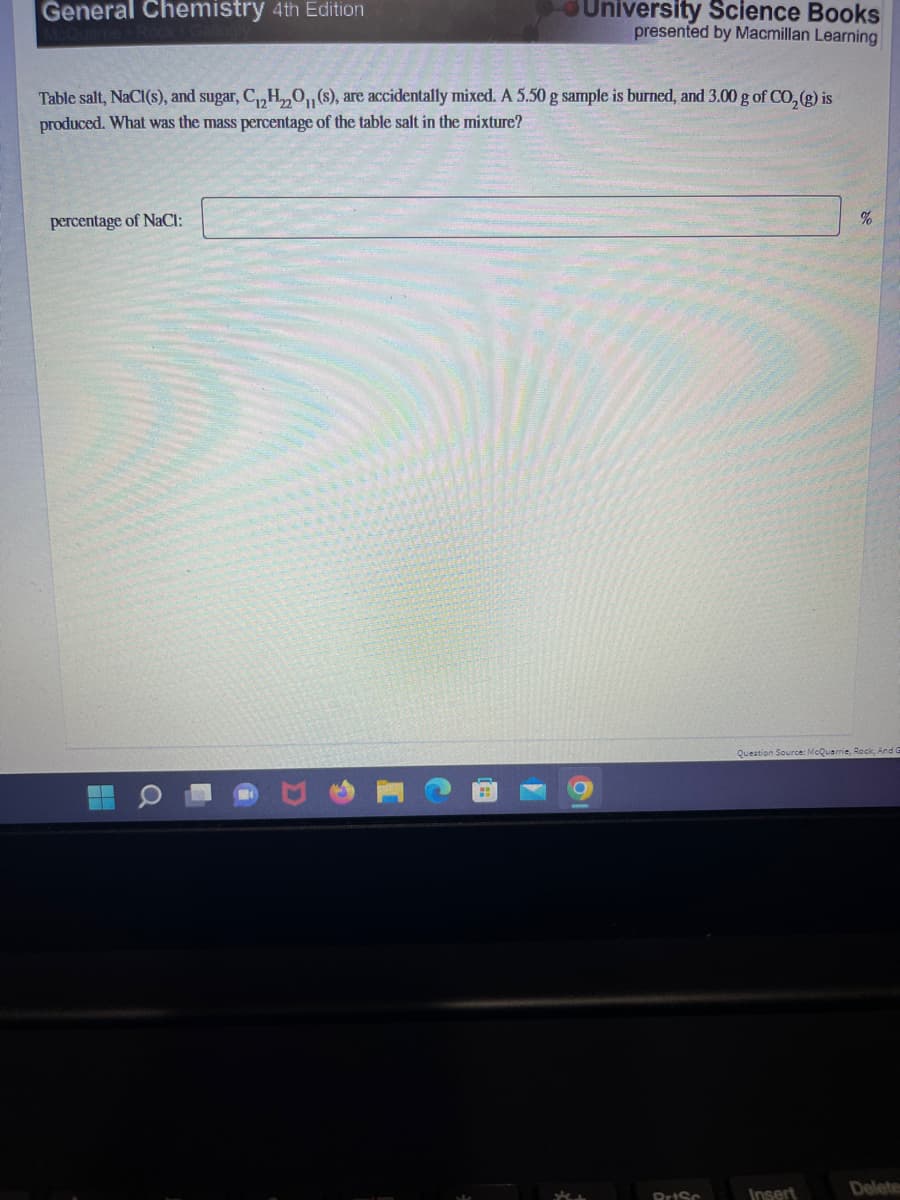
Transcribed Image Text:General Chemistry 4th Edition
Table salt, NaCl(s), and sugar, C₁₂H₂O(s), are accidentally mixed. A 5.50 g sample is burned, and 3.00 g of CO₂(g) is
produced. What was the mass percentage of the table salt in the mixture?
percentage of NaCl:
University Science Books
presented by Macmillan Learning
Q
PrtSc
%
Question Source: McQuarrie, Rock, And G
Insert
Delete
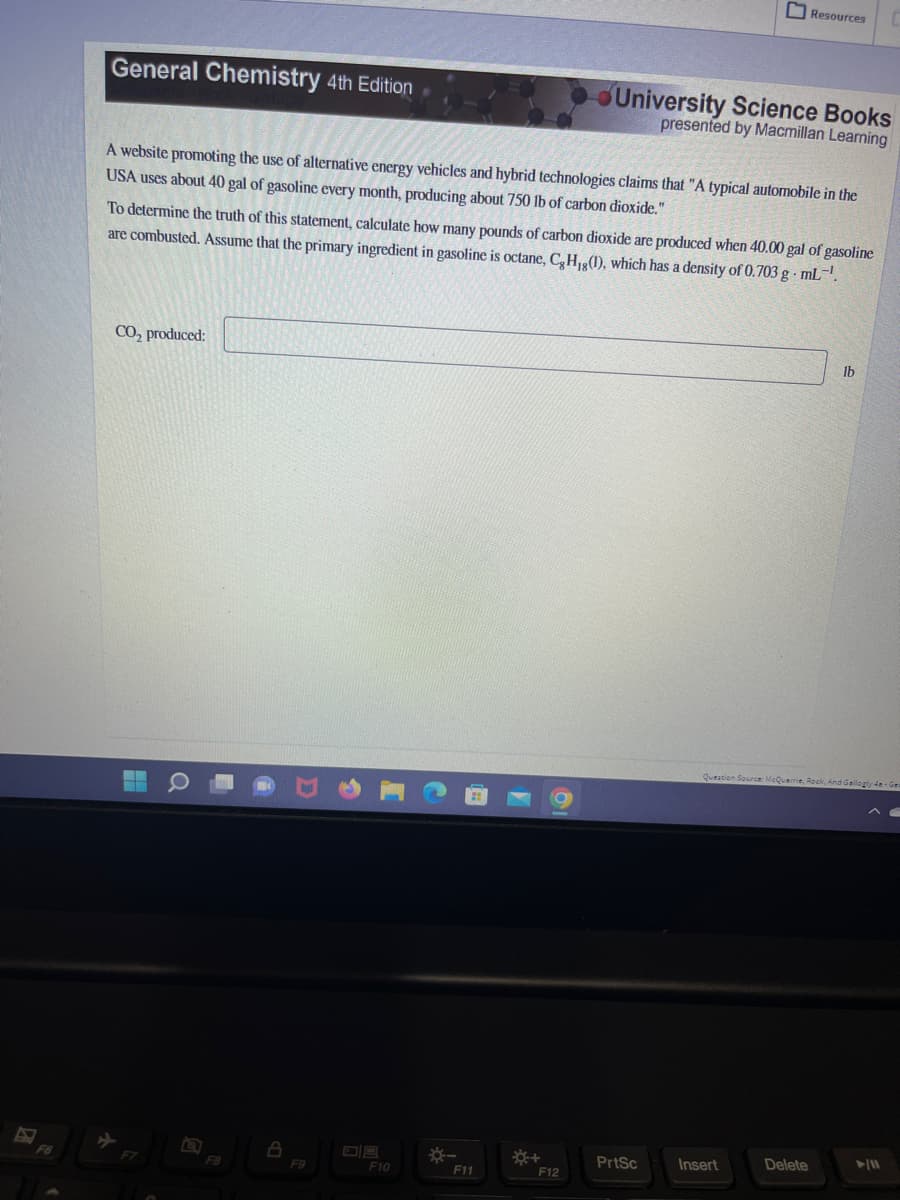
Transcribed Image Text:General Chemistry 4th Edition
A website promoting the use of alternative energy vehicles and hybrid technologies claims that "A typical automobile in the
USA uses about 40 gal of gasoline every month, producing about 750 lb of carbon dioxide."
CO₂ produced:
To determine the truth of this statement, calculate how many pounds of carbon dioxide are produced when 40.00 gal of gasoline
are combusted. Assume that the primary ingredient in gasoline is octane, C, H,g(1), which has a density of 0.703 g.mL-¹.
Q
F8
A
F9
F10
*-
F11
*+
University Science Books
presented by Macmillan Learning
F12
Resources
PrtSc
Insert
Question Source: McQuarrie, Rock, And Gallogly 4e-Ger
Delete
lb
► 11
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning