Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.19QAP
Related questions
Question
Transcribed Image Text:Pro DC
niel C. Harris - Q.. x
100%
Signatu
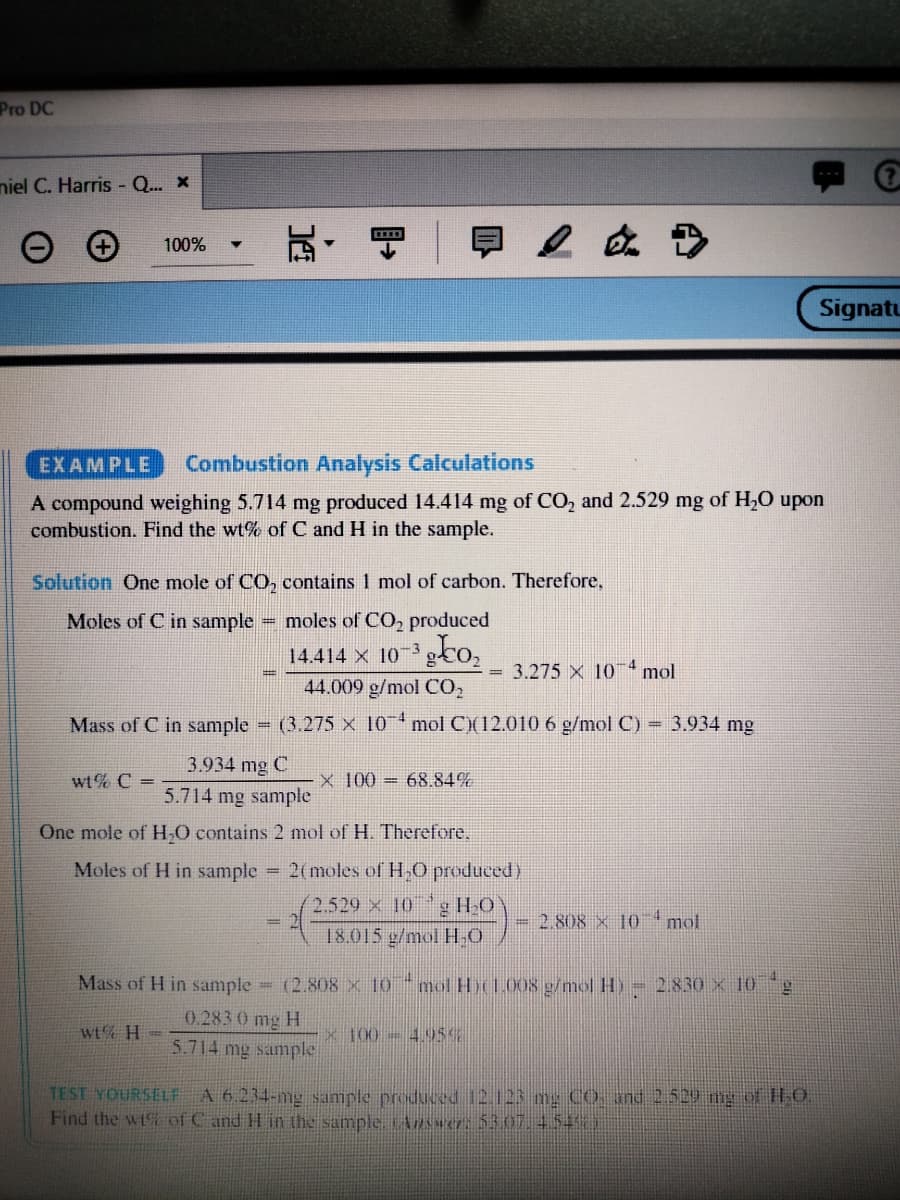
EXAMPLE
Combustion Analysis Calculations
A compound weighing 5.714 mg produced 14.414 mg of CO, and 2.529 mg of H,O upon
combustion. Find the wt% of C and H in the sample.
Solution One mole of CO, contains 1 mol of carbon. Therefore,
Moles of C in sample
moles of CO, produced
gto,
14.414 X 10-3
4
3.275 X 10
mol
44.009 g/mol CO,
Mass of C in sample
(3.275 X 10 mol C)(12.010 6 g/mol C)
= 3.934 mg
3.934 mg C
wt% C =
x 100 = 68.84%
5.714 mg sample
One mole of H,O contains 2 mol of H. Therefore.
Moles of H in sample 2(moles of H,0 produced)
g HO
18.015 g/mol HO
2.529 x 10
- 2.808 × 10mol
Mass of H in sample = (2.808 × 10 "mol H1.008 g/mol H) = 2.830 x 10
0.283 0 mg H
wt% H =
x 100-495%
5.714 mg sample
TEST YOURSELF A 6.234-mg sample produced 12.123 mg Co, and 2.529 mg of H.O
Find the wt of C and H in the sample ARwer: 5307.4.54
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
