Show ALL calculations pls. Don't reject if you dont know how to answer I need this. a. What are the average values for vph and vmo? Include calculation of net volume and errors b. What is/are the component/s of the soda ash sample? c. Calculate the percent composition of the component/s. No need to include error propagation, but you still need to apply Significant Figure rules.
Show ALL calculations pls. Don't reject if you dont know how to answer I need this. a. What are the average values for vph and vmo? Include calculation of net volume and errors b. What is/are the component/s of the soda ash sample? c. Calculate the percent composition of the component/s. No need to include error propagation, but you still need to apply Significant Figure rules.
Chapter13: Titrations In Analytical Chemistry
Section: Chapter Questions
Problem 13.28QAP
Related questions
Question
Show ALL calculations pls. Don't reject if you dont know how to answer I need this.
a. What are the average values for vph and vmo? Include calculation of net volume and errors
b. What is/are the component/s of the soda ash sample?
c. Calculate the percent composition of the component/s. No need to include error propagation, but you still need to apply Significant Figure rules.
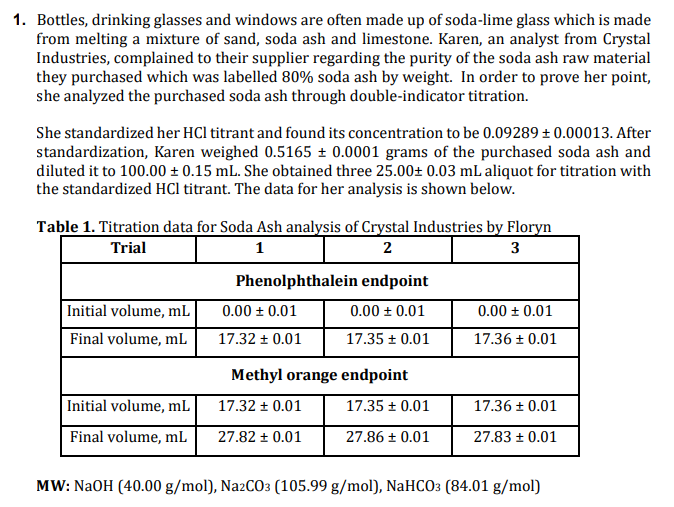
Transcribed Image Text:1. Bottles, drinking glasses and windows are often made up of soda-lime glass which is made
from melting a mixture of sand, soda ash and limestone. Karen, an analyst from Crystal
Industries, complained to their supplier regarding the purity of the soda ash raw material
they purchased which was labelled 80% soda ash by weight. In order to prove her point,
she analyzed the purchased soda ash through double-indicator titration.
She standardized her HCl titrant and found its concentration to be 0.09289 + 0.00013. After
standardization, Karen weighed 0.5165 ± 0.0001 grams of the purchased soda ash and
diluted it to 100.00 ± 0.15 mL. She obtained three 25.00± 0.03 mL aliquot for titration with
the standardized HCl titrant. The data for her analysis is shown below.
Table 1. Titration data for Soda Ash analysis of Crystal Industries by Floryn
Trial
1
3
Phenolphthalein endpoint
Initial volume, mL
0.00 + 0.01
0.00 ± 0.01
0.00 ± 0.01
Final volume, mL
17.32 ± 0.01
17.35 + 0.01
17.36 + 0.01
Methyl orange endpoint
Initial volume, mL
17.32 ± 0.01
17.35 + 0.01
17.36 + 0.01
Final volume, mL
27.82 ± 0.01
27.86 + 0.01
27.83 + 0.01
MW: NaOH (40.00 g/mol), Na2CO3 (105.99 g/mol), NAHCO3 (84.01 g/mol)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning



Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
