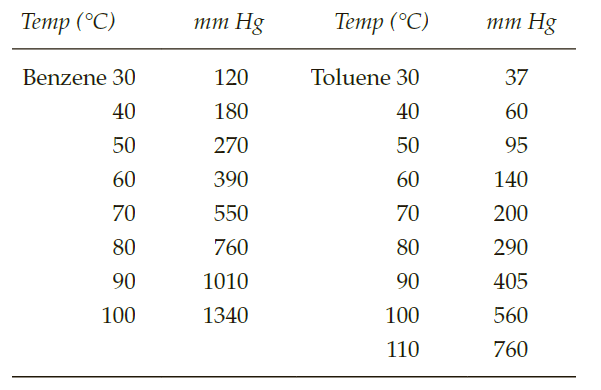
Тетр (°C) тm Hg Тетp (°С) тm Hg Benzene 30 120 Toluene 30 37 40 180 40 60 50 270 50 95 60 390 60 140 70 550 70 200 80 760 80 290 90 1010 90 405 100 1340 100 560 110 760
In the accompanying chart are approximate vapor pressures for benzene and toluene at various temperatures.
a. What is the mole fraction of each component if 3.9 g of benzene C6H6 is dissolved in 4.6 g of toluene C7H8?
b. Assuming that this mixture is ideal—that is, it follows Raoult’s Law—what
is the partial vapor pressure of benzene in this mixture at 50°C?
c. Estimate to the nearest degree the temperature at which the vapor pressure of the solution equals 1 atm (bp of the solution).
d. Calculate the composition of the vapor (mole fraction of each component) that is in equilibrium in the solution at the boiling point of this solution.
e. Calculate the composition in weight percentage of the vapor that is in equilibrium with the solution.
Trending now
This is a popular solution!
Step by step
Solved in 7 steps









