The vapor pressure of several solutions of water-propanol (C3H;OH) were determined at various compositions, and the following data of vapor pressure were collected at 45°C. Vapor Pressure of Solution (torr) Mole fraction of H20 XH20- 74.0 0.37 80.2 0.54 81.6 0.69 80.6 1.00 72.0 (a) Calculate the vapor pressure at 45°C for a solution that contains 46.0% of propanol and 54.0% water, by mole, assuming an ideal solution. (b) Determine whether water and propanol form an ideal solution, non-ideal solution with positive deviation, or non-ideal solution with negative deviation. (c) Are the overall intermolecular forces in the mixture stronger or weaker than those in pure liquids combined? Explain. (d) Which liquid has a higher boiling point, water or propanol? Explain. (e) For the three mixtures shown in the table, which one is expected to have the lowest boiling point? Explain.
The vapor pressure of several solutions of water-propanol (C3H;OH) were determined at various compositions, and the following data of vapor pressure were collected at 45°C. Vapor Pressure of Solution (torr) Mole fraction of H20 XH20- 74.0 0.37 80.2 0.54 81.6 0.69 80.6 1.00 72.0 (a) Calculate the vapor pressure at 45°C for a solution that contains 46.0% of propanol and 54.0% water, by mole, assuming an ideal solution. (b) Determine whether water and propanol form an ideal solution, non-ideal solution with positive deviation, or non-ideal solution with negative deviation. (c) Are the overall intermolecular forces in the mixture stronger or weaker than those in pure liquids combined? Explain. (d) Which liquid has a higher boiling point, water or propanol? Explain. (e) For the three mixtures shown in the table, which one is expected to have the lowest boiling point? Explain.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter11: Properties Of Solutions
Section: Chapter Questions
Problem 70E: The vapor pressures of several solutions of water-propanol (CH3CH2CH2OH) were determined at various...
Related questions
Question
100%
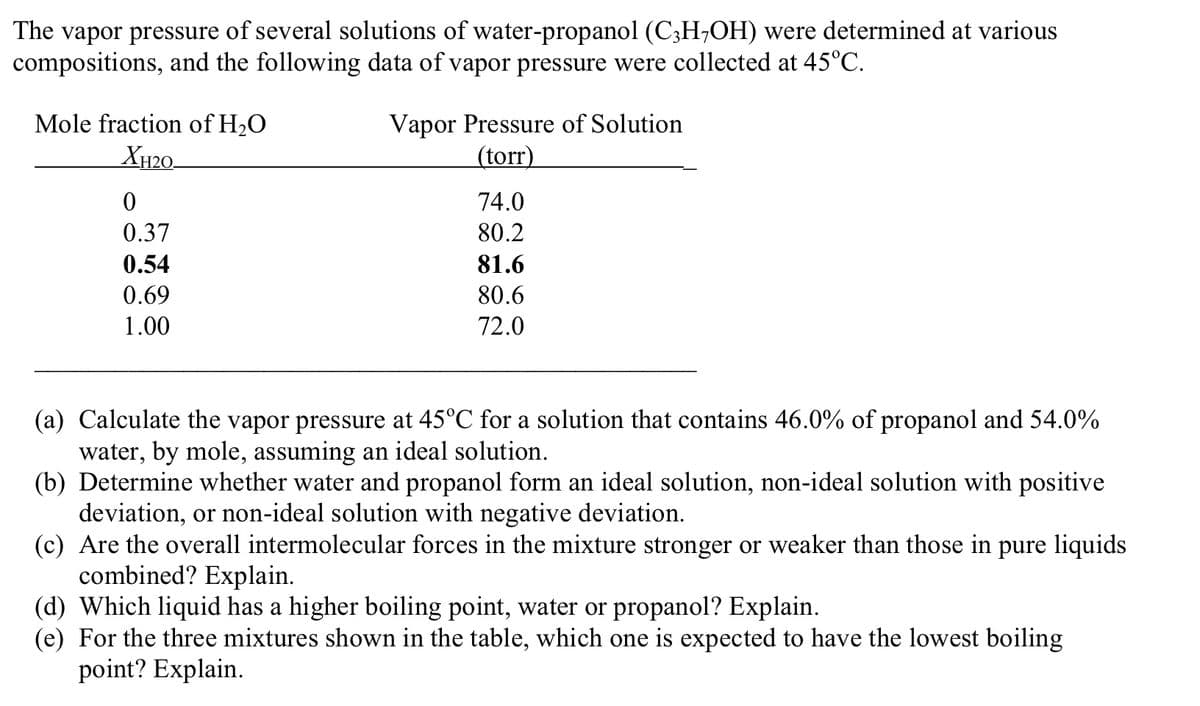
Transcribed Image Text:The vapor pressure of several solutions of water-propanol (C;H;OH) were determined at various
compositions, and the following data of vapor pressure were collected at 45°C.
Mole fraction of H2O
Vapor Pressure of Solution
(torr)
XH20-
74.0
0.37
80.2
0.54
81.6
0.69
80.6
1.00
72.0
(a) Calculate the vapor pressure at 45°C for a solution that contains 46.0% of propanol and 54.0%
water, by mole, assuming an ideal solution.
(b) Determine whether water and propanol form an ideal solution, non-ideal solution with positive
deviation, or non-ideal solution with negative deviation.
(c) Are the overall intermolecular forces in the mixture stronger or weaker than those in pure liquids
combined? Explain.
(d) Which liquid has a higher boiling point, water or propanol? Explain.
(e) For the three mixtures shown in the table, which one is expected to have the lowest boiling
point? Explain.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning