Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter9: Covalent Bonding: Orbitals
Section: Chapter Questions
Problem 68AE: The antibiotic thiarubin-A was discovered by studying the feeding habits of wild chimpanzees in...
Related questions
Question
100%
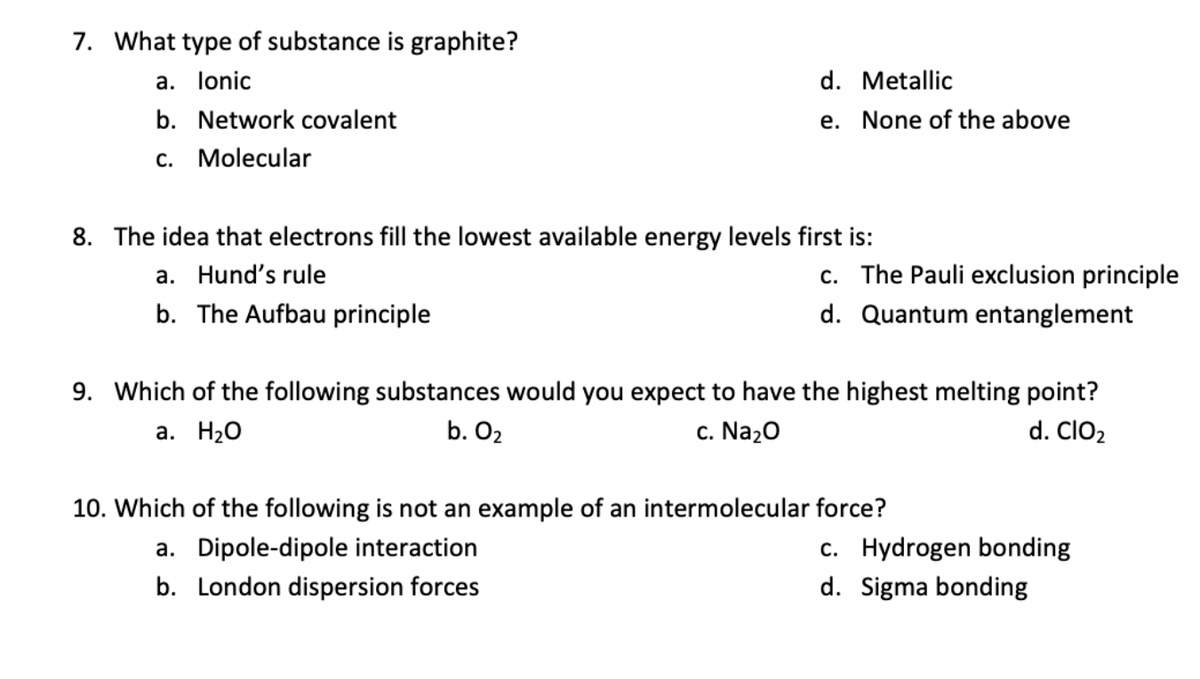
Transcribed Image Text:7. What type of substance is graphite?
a. lonic
d. Metallic
b. Network covalent
e. None of the above
c. Molecular
8. The idea that electrons fill the lowest available energy levels first is:
c. The Pauli exclusion principle
a. Hund's rule
b. The Aufbau principle
d. Quantum entanglement
9. Which of the following substances would you expect to have the highest melting point?
а. Н2О
b. O2
c. Na20
d. ClO2
10. Which of the following is not an example of an intermolecular force?
c. Hydrogen bonding
d. Sigma bonding
a. Dipole-dipole interaction
b. London dispersion forces

Transcribed Image Text:5. The 4s orbital has the shape of:
a. A sphere
C. An egg
b. A dumb-bell
d. Two perpendicular dumb-bells
6. Which of the following molecule shapes is possible when the central atom has a lone pair of electrons?
a. Trigonal pyramidal
c. Trigonal planar
b. Tetrahedral
d. Octahedral
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning