The acidity of the stomach is maintained by the H*/K* ATPase in parietal cells of the gastric mucosa. These cells have an internal pH = 7.4. The H/K ATPase transports H* across the cellular membrane into the stomach where pH = 0.8. (a) Given a membrane potential of 65 mV (inside negative), calculate AG in kJ/mol for the transport of H* into the stomach at 37 °C. Show your work.
The acidity of the stomach is maintained by the H*/K* ATPase in parietal cells of the gastric mucosa. These cells have an internal pH = 7.4. The H/K ATPase transports H* across the cellular membrane into the stomach where pH = 0.8. (a) Given a membrane potential of 65 mV (inside negative), calculate AG in kJ/mol for the transport of H* into the stomach at 37 °C. Show your work.
Human Physiology: From Cells to Systems (MindTap Course List)
9th Edition
ISBN:9781285866932
Author:Lauralee Sherwood
Publisher:Lauralee Sherwood
Chapter11: The Blood
Section: Chapter Questions
Problem 1SQE
Related questions
Question
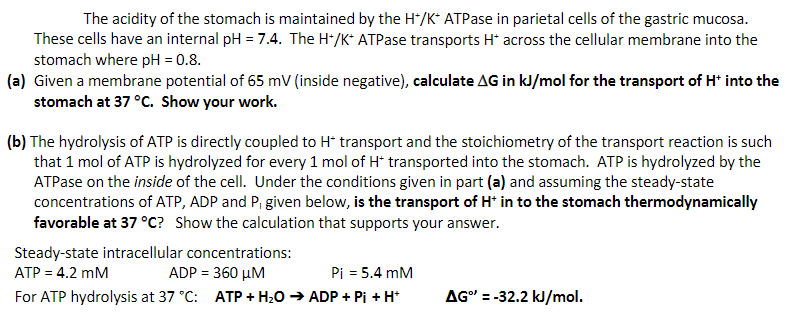
Transcribed Image Text:The acidity of the stomach is maintained by the H*/K* ATPase in parietal cells of the gastric mucosa.
These cells have an internal pH = 7.4. The H*/K* ATPase transports H* across the cellular membrane into the
stomach where pH = 0.8.
(a) Given a membrane potential of 65 mV (inside negative), calculate AG in kJ/mol for the transport of H* into the
stomach at 37 °C. Show your work.
(b) The hydrolysis of ATP is directly coupled to H* transport and the stoichiometry of the transport reaction is such
that 1 mol of ATP is hydrolyzed for every 1 mol of H* transported into the stomach. ATP is hydrolyzed by the
ATPase on the inside of the cell. Under the conditions given in part (a) and assuming the steady-state
concentrations of ATP, ADP and P₁ given below, is the transport of H* in to the stomach thermodynamically
favorable at 37 °C? Show the calculation that supports your answer.
Steady-state intracellular concentrations:
ATP = 4.2 mM
ADP = 360 μM
Pi = 5.4 mM
For ATP hydrolysis at 37 °C: ATP + H₂O → ADP + Pi + H+
AG" = -32.2 kJ/mol.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Human Physiology: From Cells to Systems (MindTap …
Biology
ISBN:
9781285866932
Author:
Lauralee Sherwood
Publisher:
Cengage Learning

Human Physiology: From Cells to Systems (MindTap …
Biology
ISBN:
9781285866932
Author:
Lauralee Sherwood
Publisher:
Cengage Learning