The activity coefficient of Ni²+ ion (a Ni2* =0.6 nm) in a solution that has an ionic strength of 0.055 is: 0.51ziV 1 + 3.3ax Vu -log Yx = %3D Please fill in the space with a numerical value with three digits or decimal places without units
The activity coefficient of Ni²+ ion (a Ni2* =0.6 nm) in a solution that has an ionic strength of 0.055 is: 0.51ziV 1 + 3.3ax Vu -log Yx = %3D Please fill in the space with a numerical value with three digits or decimal places without units
Chapter10: Effect Of Electrolytes On Chemical Equilibria
Section: Chapter Questions
Problem 10.5QAP
Related questions
Question
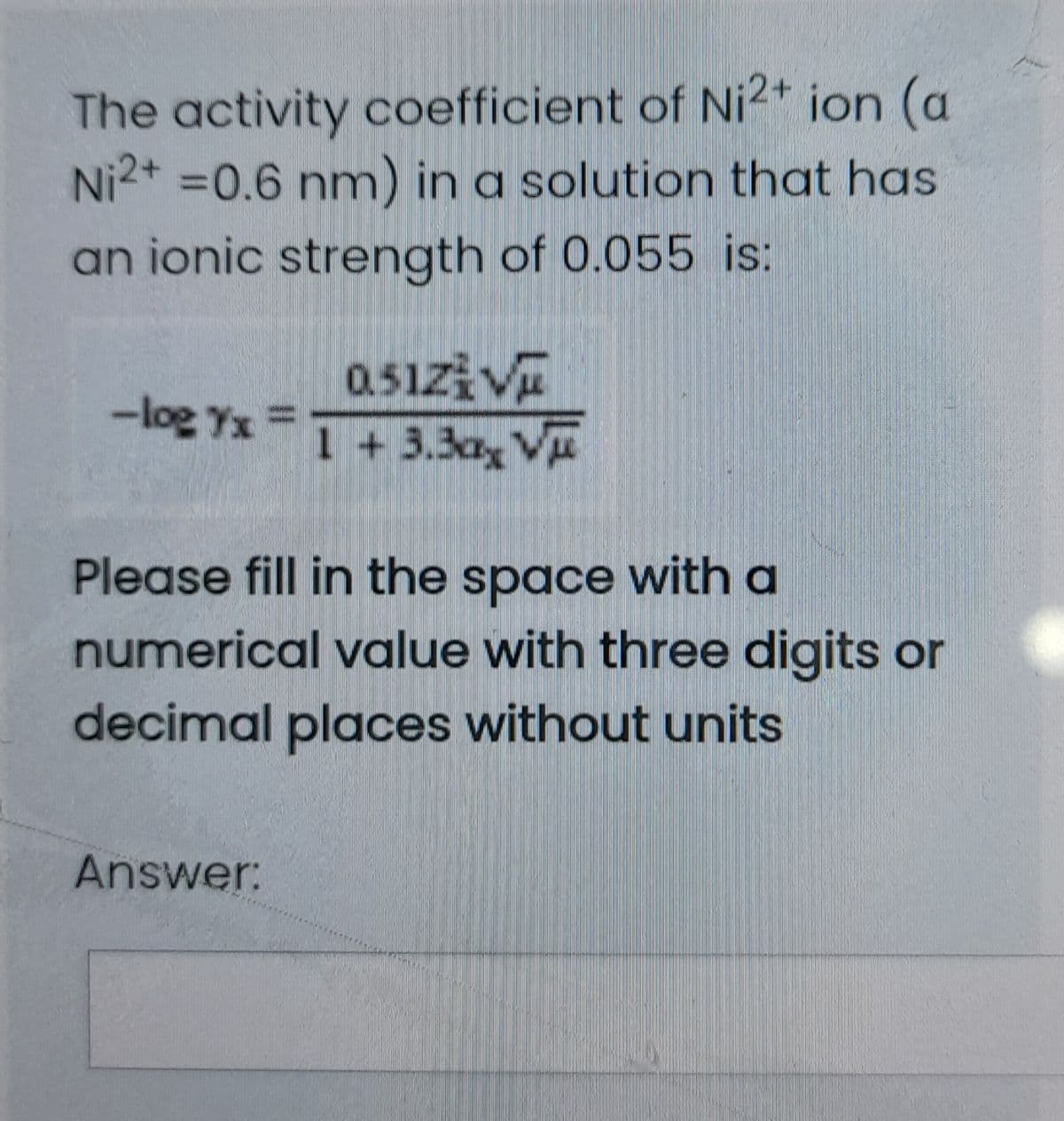
Transcribed Image Text:The activity coefficient of Ni2+ ion (a
Ni2+ =0.6 nm) in a solution that has
an ionic strength of 0.055 is:
0.51zi Vu
1 + 3.3ax V
-log Yx =
Please fill in the space with a
numerical value with three digits or
decimal places without units
Answer:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

