Calculate the [Ag*] of a saturated solution of Agl that has an ionic strength of 0.10 M using activity coefficients. Ksp (Agl) = 8.3 x 10-17 Species Ag* H3O* OH Activity Coefficient, y, at 0.10 M 0.75 0.755 0.83 0.76 Please fill in the blanks for the number and exponent. For example if the answer is 1.1 x 102 input "1.1" in the first box followed by -2 in the second box.
Calculate the [Ag*] of a saturated solution of Agl that has an ionic strength of 0.10 M using activity coefficients. Ksp (Agl) = 8.3 x 10-17 Species Ag* H3O* OH Activity Coefficient, y, at 0.10 M 0.75 0.755 0.83 0.76 Please fill in the blanks for the number and exponent. For example if the answer is 1.1 x 102 input "1.1" in the first box followed by -2 in the second box.
Chapter8: Polyfunctional Acids And Bases
Section: Chapter Questions
Problem 10P
Related questions
Question
![Calculate the [Ag*] of a saturated solution of Agl that has an ionic strength of 0.10 M using
activity coefficients.
Ksp (Agl) = 8.3 x 10-17
Species
Ag*
H3O*
OH
Activity Coefficient, y, at 0.10 M
0.75 0.755
0.83
0.76
Please fill in the blanks for the number and exponent. For example if the answer is 1.1 x 10-2
input "1.1" in the first box followed by -2 in the second box.
[Ag*] =
х 10
%3D](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F2f591c64-216a-4120-ac44-1b23dfa641af%2Fc2541016-566c-4232-9794-d3d34f6f8789%2Forz7jmk_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Calculate the [Ag*] of a saturated solution of Agl that has an ionic strength of 0.10 M using
activity coefficients.
Ksp (Agl) = 8.3 x 10-17
Species
Ag*
H3O*
OH
Activity Coefficient, y, at 0.10 M
0.75 0.755
0.83
0.76
Please fill in the blanks for the number and exponent. For example if the answer is 1.1 x 10-2
input "1.1" in the first box followed by -2 in the second box.
[Ag*] =
х 10
%3D
Expert Solution
Step 1
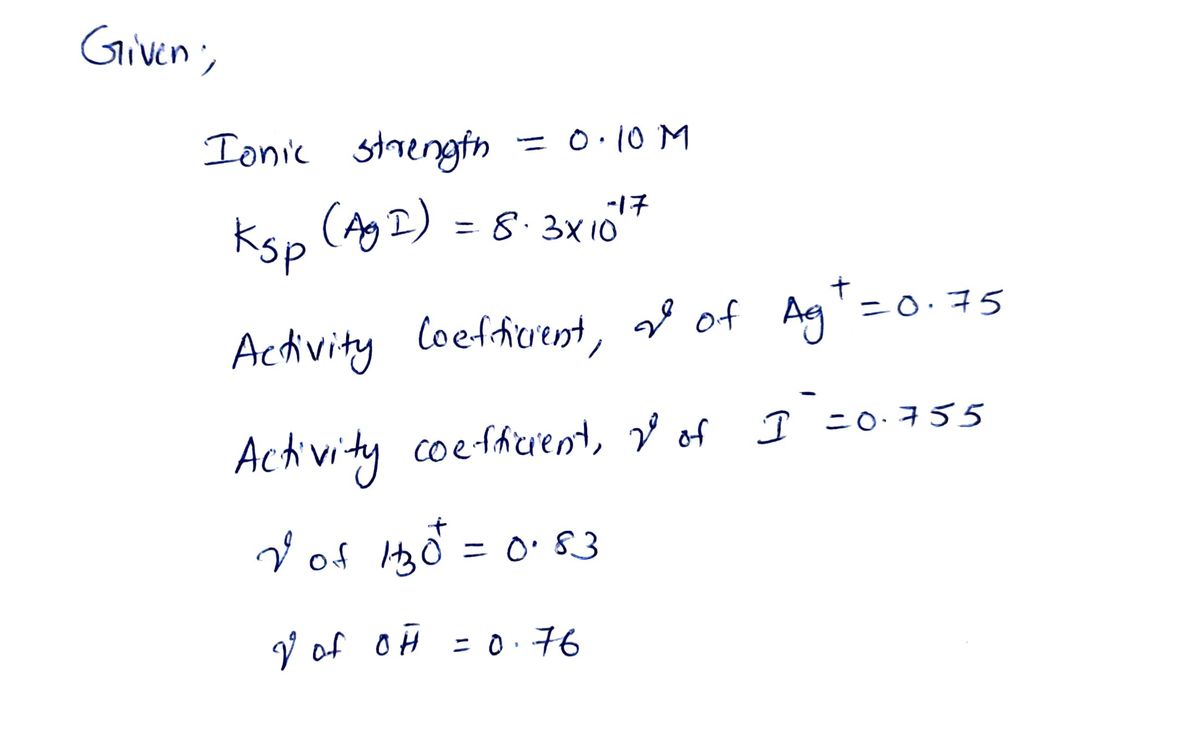
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning



Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning
