Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter17: Spontaneity, Entropy, And Free Energy
Section: Chapter Questions
Problem 128IP: Some nonelectrolyte solute (molar mass = 142 g/mol) was dissolved in 150. mL of a solvent (density =...
Related questions
Question
The boiling point of an aqueous solution is 101.45 Celsius. What is the freezing point?
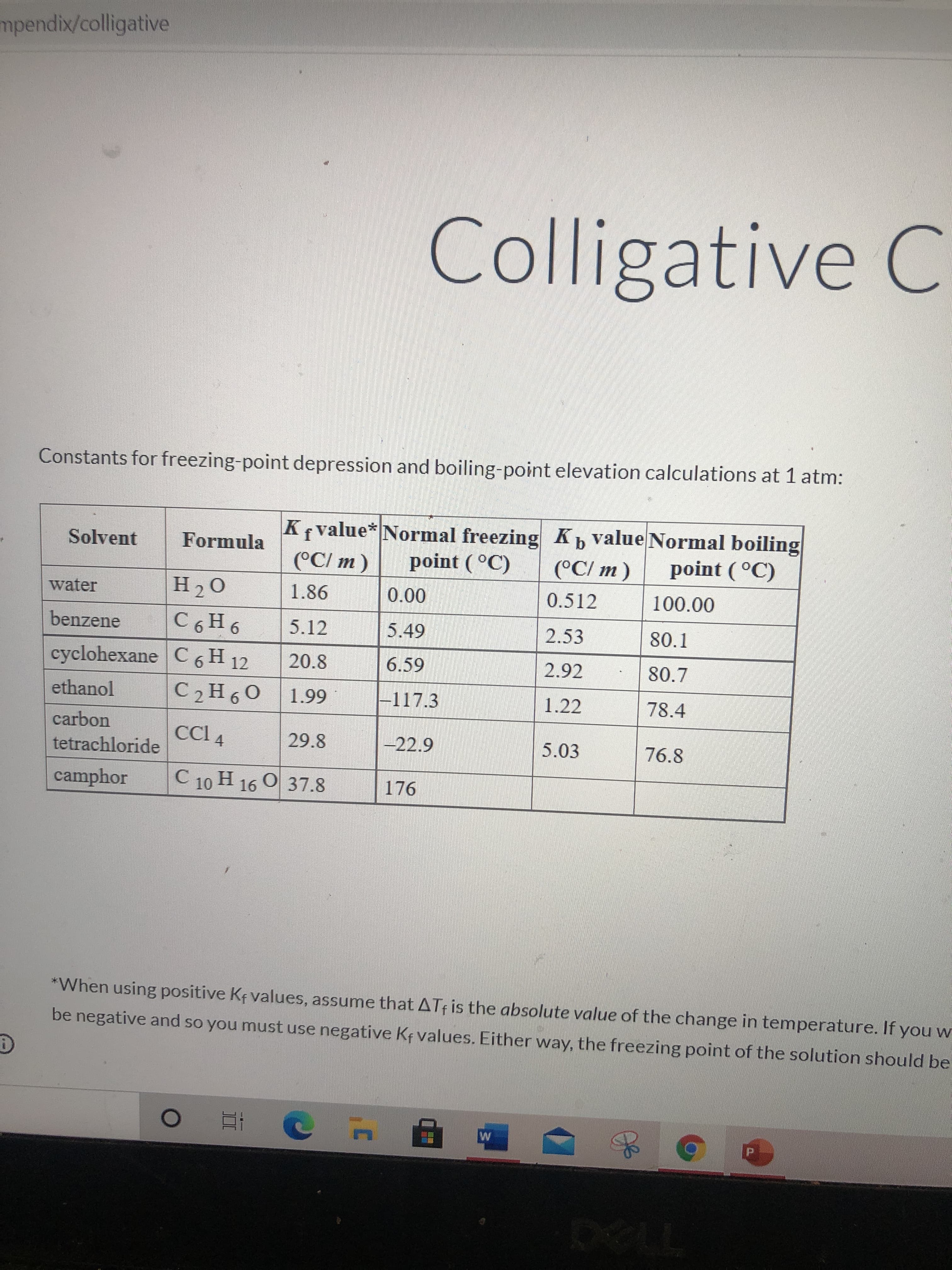
Transcribed Image Text:mpendix/colligative
Colligative C
31
Constants for freezing-point depression and boiling-point elevation calculations at 1 atm:
K value* Normal freezing K value Normal boiling
(°C/ m)
Solvent
Formula
point ( °C)
(°C/ m)
point ( °C)
water
H20
1.86
00 0
5.49
0.512
100.00
9 H9Ɔ
20.8
benzene
5.12
2.53
80.1
cyclohexane C6 H 12
2.92
80.7
ethanol
66
-117.3
1.22
78.4
9.
carbon
CCI 4
29.8
-22.9
5.03
76.8
tetrachloride
camphor
C.
C 10 H 16 O 37.8
*When using positive Kf values, assume that ATf is the absolute value of the change in temperature. If you w
be negative and so you must use negative Kf values. Either way, the freezing point of the solution should be
近。
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,