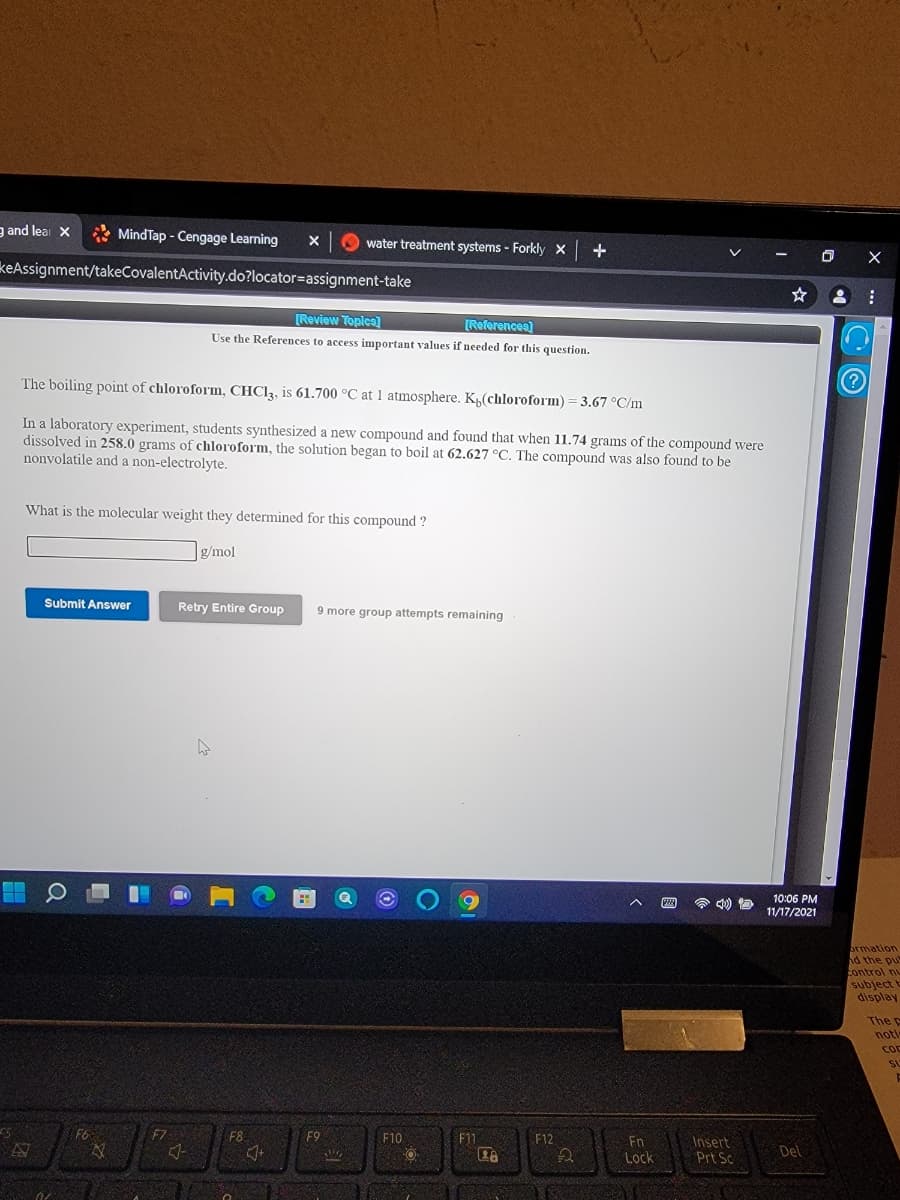
The boiling point of chloroform, CHC13, is 61.700 °C at 1 atmosphere. K(chloroform) = 3.67 °C/m In a laboratory experiment, students synthesized a new compound and found that when 11.74 grams of the compound were dissolved in 258.0 grams of chloroform, the solution began to boil at 62.627 °C. The compound was also found to be nonvolatile and a non-electrolyte.
The boiling point of chloroform, CHC13, is 61.700 °C at 1 atmosphere. K(chloroform) = 3.67 °C/m In a laboratory experiment, students synthesized a new compound and found that when 11.74 grams of the compound were dissolved in 258.0 grams of chloroform, the solution began to boil at 62.627 °C. The compound was also found to be nonvolatile and a non-electrolyte.
Chapter7: Solutions And Colloids
Section: Chapter Questions
Problem 7.89E
Related questions
Question
Transcribed Image Text:g and lear X
* MindTap - Cengage Learning
O water treatment systems - Forkly x
keAssignment/takeCovalentActivity.do?locator=assignment-take
:
[Review Topica)
[References)
Use the References to access important values if needed for this question.
The boiling point of chloroform, CHC13, is 61.700 °C at 1 atmosphere. Kµ(chloroform) = 3.67 °C/m
In a laboratory experiment, students synthesized a new compound and found that when 11.74 grams of the compound were
dissolved in 258.0 grams of chloroform, the solution began to boil at 62.627 °C. The compound was also found to be
nonvolatile and a non-electrolyte.
What is the molecular weight they determined for this compound ?
g/mol
Submit Answer
Retry Entire Group
9 more group attempts remaining
10:06 PM
11/17/2021
brmation
d the pu
control nu
subject
display
The p
noti
COD
F6
F7
F8
F9
F10
F1
F12
Fn
Lock
Insert
Prt Sc
28
Del
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



