The chemical reaction shown was performed and the concentration of CCl4CCl4 was measured over time. Cl2(g)+CHCl3(g)⟶HCl(g)+CCl4(g)Cl2(g)+CHCl3(g)⟶HCl(g)+CCl4(g) The [CCl4]after 9 s was 0.049 mol/L. After 138 s, the [CCl4] was 0.451 mol/L. Calculate the rate of reaction.
The chemical reaction shown was performed and the concentration of CCl4CCl4 was measured over time. Cl2(g)+CHCl3(g)⟶HCl(g)+CCl4(g)Cl2(g)+CHCl3(g)⟶HCl(g)+CCl4(g) The [CCl4]after 9 s was 0.049 mol/L. After 138 s, the [CCl4] was 0.451 mol/L. Calculate the rate of reaction.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter11: Rate Of Reaction
Section: Chapter Questions
Problem 92QAP: Consider the decomposition reaction 2X2Y+ZThe following graph shows the change in concentration with...
Related questions
Question
The
Cl2(g)+CHCl3(g)⟶HCl(g)+CCl4(g)Cl2(g)+CHCl3(g)⟶HCl(g)+CCl4(g)
The [CCl4]after 9 s was 0.049 mol/L. After 138 s, the [CCl4] was 0.451 mol/L. Calculate the
Expert Solution
Step 1
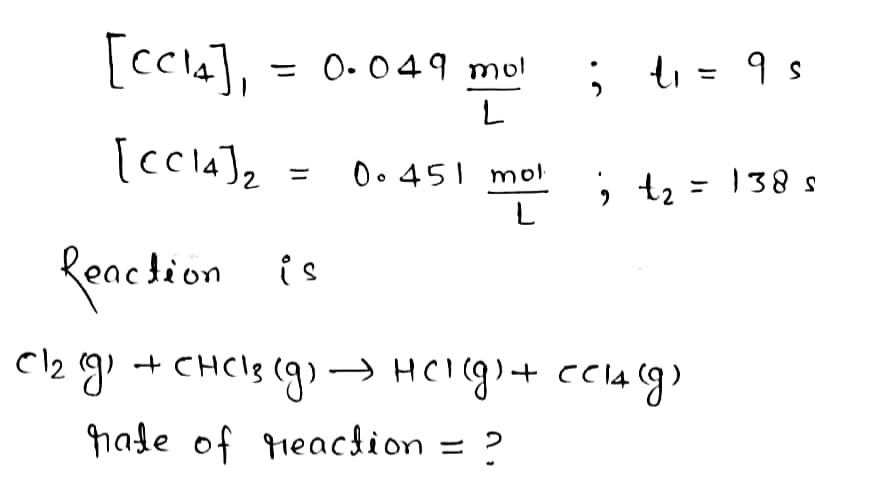
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning