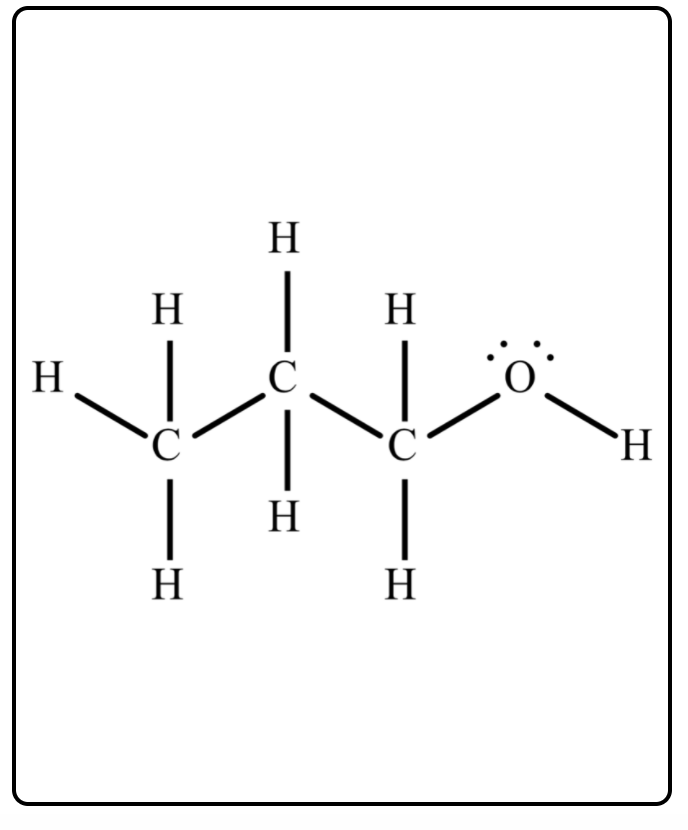
The compounds 1-propanol, CH₃CH₂CH₂OH, and ethyl methyl ether, CH₃CH₂OCH₃ have the same chemical formula. a)Based on the chemical structure shown, what intermolecular forces are present in a molecule of 1-propanol? a) Dispersion forces only b) Dispersion forces and dipole-dipole interactions c) Dispersion forces and hydrogen bonding d) Dispersion forces, dipole-dipole interactions, and hydrogen bonding
The compounds 1-propanol, CH₃CH₂CH₂OH, and ethyl methyl ether, CH₃CH₂OCH₃ have the same chemical formula. a)Based on the chemical structure shown, what intermolecular forces are present in a molecule of 1-propanol? a) Dispersion forces only b) Dispersion forces and dipole-dipole interactions c) Dispersion forces and hydrogen bonding d) Dispersion forces, dipole-dipole interactions, and hydrogen bonding
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
The compounds 1-propanol, CH₃CH₂CH₂OH, and ethyl methyl ether, CH₃CH₂OCH₃ have the same chemical formula.
a)Based on the chemical structure shown, what intermolecular forces are present in a molecule of 1-propanol?
a) Dispersion forces only
b) Dispersion forces and dipole-dipole interactions
c) Dispersion forces and hydrogen bonding
d) Dispersion forces, dipole-dipole interactions, and hydrogen bonding
Transcribed Image Text:H
H
H
H
`H
H
H
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY
Expert Answers to Latest Homework Questions
Q: Consider the indefinite integral
x51 dx:
This can be transformed into a basic integral by letting
u…
Q: Write the following expression as the sine, cosine, or tangent of a double angle. Then find the…
Q: Scenario analysis is defined as the:
Multiple Choice
determination of the initial cash…
Q: Consider a triangle ABC like the one below. Suppose that a= 52, b=65, and c=14
. (The figure is not…
Q: Describe two types of persona presented by Pruitt & Adlin (2009).
Q: The Dreitz Company offered 12,000 shares in a rights offer. By prior agreement, the underwriter,…
Q: 7. Study Questions and Problems #7
Environmentalists in Tennessee brought suit against the Champion…
Q: You purchased Butterfly Wing Corporation stock exactly one year ago at a price of $77.66 per share.…
Q: Consider a triangle ABC like the one below. Suppose that
A=54°,C=50°, and b=13
. (The figure is…
Q: Your portfolio has a beta of 1.57. The portfolio consists of 16 percent U.S. Treasury bills, 35…
Q: Face recognition is increasingly adopted for mobile devices. Samsung has been one of the first to…
Q: A bond that pays interest semiannually has a price of $981.73 and a semiannual coupon payment of…
Q: Crossfade Corporation has a bond with a par value of $2,000 that sells for $1,925.24. The bond has a…
Q: Present THREE methods of avoid accidental activation and ONE example for EACH method
Q: Explain the principle of “avoid accidental activation”
Q: List and explain all three classes of decision making?
Q: The cell potential between two beakers containing copper(II) sulfate solutions and copper metal…
Q: A company wanted to test whether an employee could detect
the presence of an alarm in
a certain…
Q: A current of 6.78×104 A is passed through an electrolysis cell containing molten KCI for 13.9 days.…
Q: A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction:
Cu2+…
Q: 2. Study Questions and Problems #2
Suppose a car sells for $21,000 in a market with no pollution…