The concentration of many colorful solutions can be determined by the amount of light absorbed by the solution. The relationship between absorbance and concentration is a linear relationship known as Beer's law. The data below show the absorbance of a series of solutions of known concentrations of Substance A. Plot these data using Excel and determine the equation of the trendline. Use that equation to determine the concentration (M) of an unknown sample of Substance A. Concentration of Substance A (M) Absorbance
The concentration of many colorful solutions can be determined by the amount of light absorbed by the solution. The relationship between absorbance and concentration is a linear relationship known as Beer's law. The data below show the absorbance of a series of solutions of known concentrations of Substance A. Plot these data using Excel and determine the equation of the trendline. Use that equation to determine the concentration (M) of an unknown sample of Substance A. Concentration of Substance A (M) Absorbance
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter20: Molecular Spectroscopy And Photochemistry
Section: Chapter Questions
Problem 6P
Related questions
Question
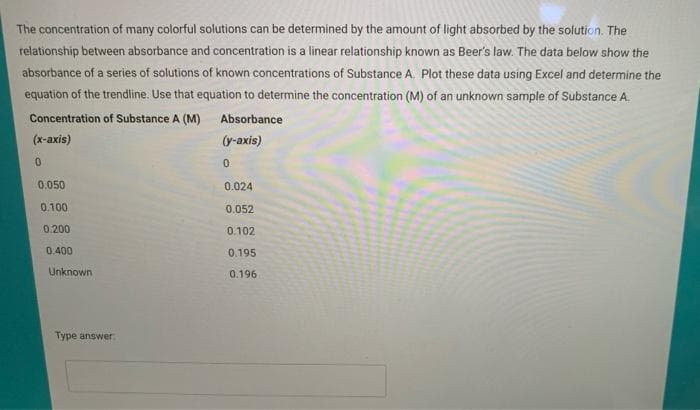
Transcribed Image Text:The concentration of many colorful solutions can be determined by the amount of light absorbed by the solution. The
relationship between absorbance and concentration is a linear relationship known as Beer's law. The data below show the
absorbance of a series of solutions of known concentrations of Substance A. Plot these data using Excel and determine the
equation of the trendline. Use that equation to determine the concentration (M) of an unknown sample of Substance A.
Concentration of Substance A (M)
Absorbance
(x-axis)
(y-axis)
0.050
0.024
0.100
0.052
0.200
0.102
0.400
0.195
Unknown
0.196
Type answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning