Mathematics For Machine Technology
8th Edition
ISBN:9781337798310
Author:Peterson, John.
Publisher:Peterson, John.
Chapter30: Customary And Metric Steel Rules
Section: Chapter Questions
Problem 17A: Decimal-Inch Steel Rules Read measurements a-d on the enlarged fractional rule shown Figure 30-16.
Related questions
Question
please help
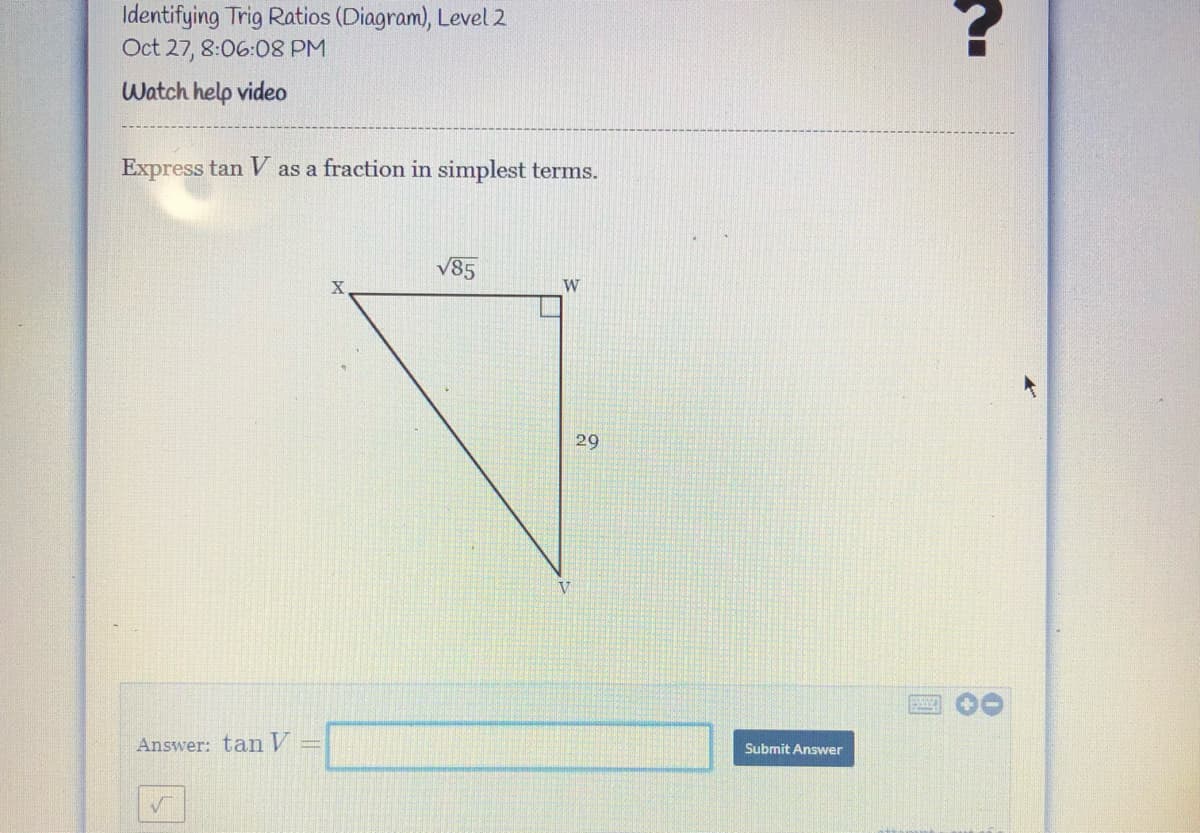
Transcribed Image Text:Identifying Trig Ratios (Diagram), Level 2
Oct 27, 8:06:08 PM
Watch help video
Express tan V as a fraction in simplest terms.
V85
W
29
V
Answer: tan V
Submit Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Please help

Transcribed Image Text:The count in a bacteria culture was 100 after 20 minutes and 1100 after 30 minutes. Assuming the count
grows exponentially,
What was the initial size of the culture?
Find the doubling period.
Find the population after 115 minutes.
When will the population reach 11000.
Solution
Follow-up Question
Please help

Transcribed Image Text:Blood is a buffer solution. When carbon dioxide is absorbed into the bloodstream, it produces carbonic acid
and lowers the pH. The body compensates by producing bicarbonate, a weak base to partially neutralize
the acid. The equation which models blood pH in this situation is
800
pН(«) — 6.1 + log
where x is the partial pressure of carbon dioxide in arterial blood, measured in torr. Find the partial
pressure of carbon dioxide in arterial blood if the pH is 7.1.
Solution
Follow-up Question
Please help

Transcribed Image Text:Find the time required for an investment of $ 5000 to grow to $ 8700 at an interest rate of 7.5 percent per
year, compounded quarterly. Round to 2 decimal places.
t :
years.
Solution
Follow-up Question
Please help

Transcribed Image Text:The half-life of Palladium-100 is 4 days. After 24 days a sample of Palladium-100 has been reduced to a
mass of 5 mg.
What was the initial mass (in mg) of the sample?
What is the mass 6 weeks after the start? Round your answer to 8 decimal places.
Solution
Follow-up Question
Please help

Transcribed Image Text:A bacteria culture initially contains 2000 bacteria and doubles every half hour. The formula for the
population is p(t)
2000e*t for some constant k. (You will need to find k to answer the following.)
||
Find the size of the baterial population after 80 minutes.
Find the size of the baterial population after 7 hours.
Solution
Follow-up Question
Please help

Transcribed Image Text:The half-life of strontium-90 is 28 years. How long will it take a 44 mg sample to decay to a mass of 11 mg?
Your answer is
years.
Solution
Follow-up Question
help

Transcribed Image Text:Some medicines can wear off in the body exponentially. In this case, assume the rate decays hourly for a
starting amount of 300 mg of medication according to the equation:
M = 300(0.87)
Solve this equation to determine the number of hours, t, that it will take for the initial 300 mg of medicine
to decrease to 30 mg.
Answer: It will take about
hours. (Round to TWO decimal places.)
Solution
Recommended textbooks for you

Mathematics For Machine Technology
Advanced Math
ISBN:
9781337798310
Author:
Peterson, John.
Publisher:
Cengage Learning,

Holt Mcdougal Larson Pre-algebra: Student Edition…
Algebra
ISBN:
9780547587776
Author:
HOLT MCDOUGAL
Publisher:
HOLT MCDOUGAL

Elementary Geometry For College Students, 7e
Geometry
ISBN:
9781337614085
Author:
Alexander, Daniel C.; Koeberlein, Geralyn M.
Publisher:
Cengage,

Mathematics For Machine Technology
Advanced Math
ISBN:
9781337798310
Author:
Peterson, John.
Publisher:
Cengage Learning,

Holt Mcdougal Larson Pre-algebra: Student Edition…
Algebra
ISBN:
9780547587776
Author:
HOLT MCDOUGAL
Publisher:
HOLT MCDOUGAL

Elementary Geometry For College Students, 7e
Geometry
ISBN:
9781337614085
Author:
Alexander, Daniel C.; Koeberlein, Geralyn M.
Publisher:
Cengage,