The cylinder in Egure 1) has a moveable peston attached to a pring The cylinder's cross-section area is 10 cm², it contains 0050 mol of gas, and the spring constant is 1500 N/m At 15°C ne spring is neither compressed nor stretched ure A-10 cm 1500 N/m www 1 of 1 How far is the spring compressed if the gas temperature is raised to 150°C Express your answer to two significant figures and include the appropriate units. View Available Hint(s) Ar 3.16 Submit HA Provide Feedback 4 Previous Answers → cm X Incorrect; Try Again; 2 attempts remaining
The cylinder in Egure 1) has a moveable peston attached to a pring The cylinder's cross-section area is 10 cm², it contains 0050 mol of gas, and the spring constant is 1500 N/m At 15°C ne spring is neither compressed nor stretched ure A-10 cm 1500 N/m www 1 of 1 How far is the spring compressed if the gas temperature is raised to 150°C Express your answer to two significant figures and include the appropriate units. View Available Hint(s) Ar 3.16 Submit HA Provide Feedback 4 Previous Answers → cm X Incorrect; Try Again; 2 attempts remaining
Chapter3: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 56P: Consider a cylinder with a movable piston containing n moles of an ideal gas. The entire apparatus...
Related questions
Question
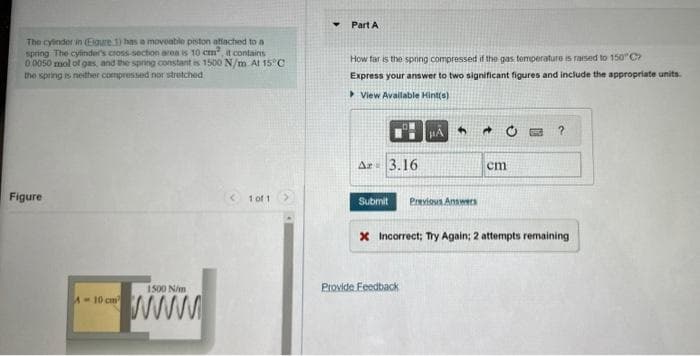
Transcribed Image Text:The cylinder in (Egure 1) has a moveable piston attached to a
spring The cylinder's cross-section area is 10 cm², it contains
0 0050 mol of gas, and the spring constant is 1500 N/m At 15°C
the spring is neither compressed nor stretched
Figure
1500 N/m
www.
A-10 cm
< 1 of 1
Y Part A
How far is the spring compressed if the gas temperature is raised to 150°C
Express your answer to two significant figures and include the appropriate units.
View Available Hint(s)
Ar 3.16
4
#A
Submit Previous Answers
Provide Feedback
→
cm
?
x Incorrect; Try Again; 2 attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University


University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University