Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 25E: At 40C, H2O2 (aq) will decompose according to the following reaction: 2H2O2(aq)2H2O(l)+O2(g) The...
Related questions
Question

Transcribed Image Text:2. Give the form of the rate law for this reaction (including orders). Also give the overall order
for this reaction:
rate law:
overall order =
3. Calculate the rate constant, k, for this reaction (using the data from Experiment #1. Show all
your work):
4. Explain exactly what experiment(s) must be performed in order to evaluate the activation
energy (E) for this reaction. What data must be plotted?
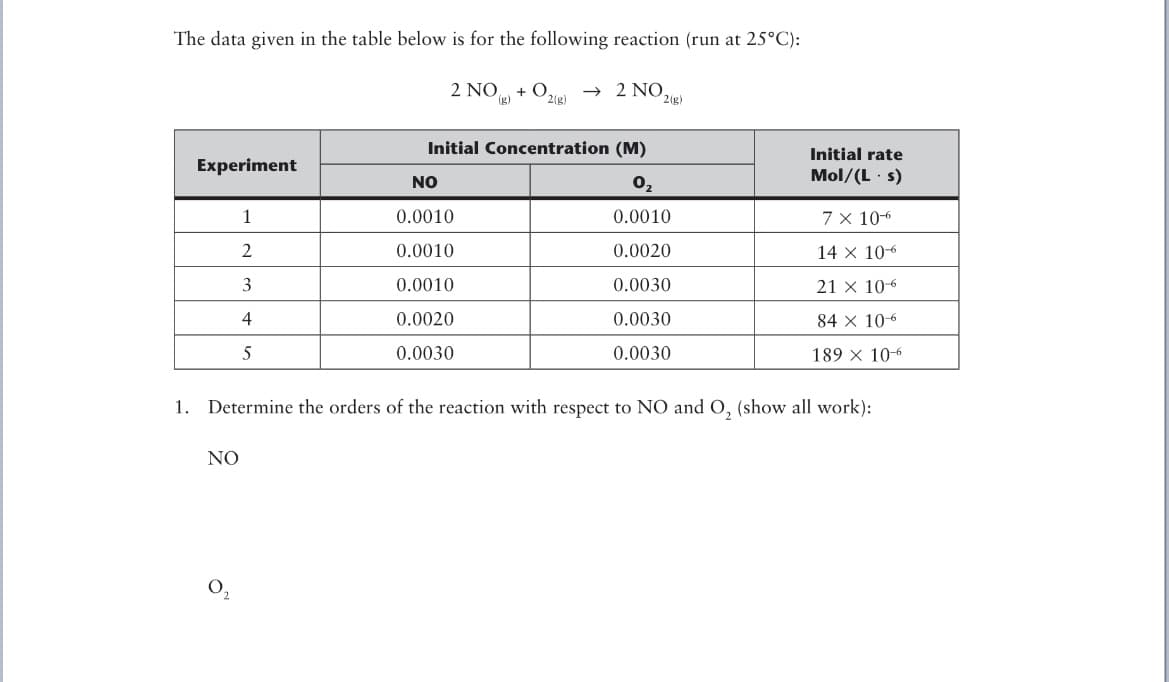
Transcribed Image Text:The data given in the table below is for the following reaction (run at 25°C):
2 NO
+ O,
2(g)
→ 2
(g)
2(g)
Initial Concentration (M)
Initial rate
Experiment
NO
0,
Mol/(L · s)
1
0.0010
0.0010
7 x 10-6
2
0.0010
0.0020
14 x 106
3
0.0010
0.0030
21 x 10-6
4
0.0020
0.0030
84 x 10-6
5
0.0030
0.0030
189 x 106
1. Determine the orders of the reaction with respect to NO and O, (show all work):
NO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning