The details of a chemical reaction between A and B, to produce the product C, is shown below. Use these data to answer items 25 to 30. Data 1 States: A = aqueous B = solid C = gas D = aqueous A + B Data 2 B. Data 2 C. Data 3 D. Data 1 and 2 C + 25. Which data provides information about the kinetics of the reaction? A. Data 1 B. Data 2 C. Data 3 D. Data 2 and 3 Ea AG 26. In what data do you see the qualitative description of the forming and breaking of intermolecular forces of attraction between the reactants? A. Data 1 27. Which of the following descriptions is TRUE for the chemical reaction shown above? A. The reaction is classified as an exothermic reaction. B. The products will decrease if the reactants increase. C. The reaction is reversible if you add more heat. D. The reaction changes to exothermic reaction if heat is increased. Data 3
The details of a chemical reaction between A and B, to produce the product C, is shown below. Use these data to answer items 25 to 30. Data 1 States: A = aqueous B = solid C = gas D = aqueous A + B Data 2 B. Data 2 C. Data 3 D. Data 1 and 2 C + 25. Which data provides information about the kinetics of the reaction? A. Data 1 B. Data 2 C. Data 3 D. Data 2 and 3 Ea AG 26. In what data do you see the qualitative description of the forming and breaking of intermolecular forces of attraction between the reactants? A. Data 1 27. Which of the following descriptions is TRUE for the chemical reaction shown above? A. The reaction is classified as an exothermic reaction. B. The products will decrease if the reactants increase. C. The reaction is reversible if you add more heat. D. The reaction changes to exothermic reaction if heat is increased. Data 3
Chapter12: Gravimetric Methods Of Analysis
Section: Chapter Questions
Problem 12.26QAP
Related questions
Question
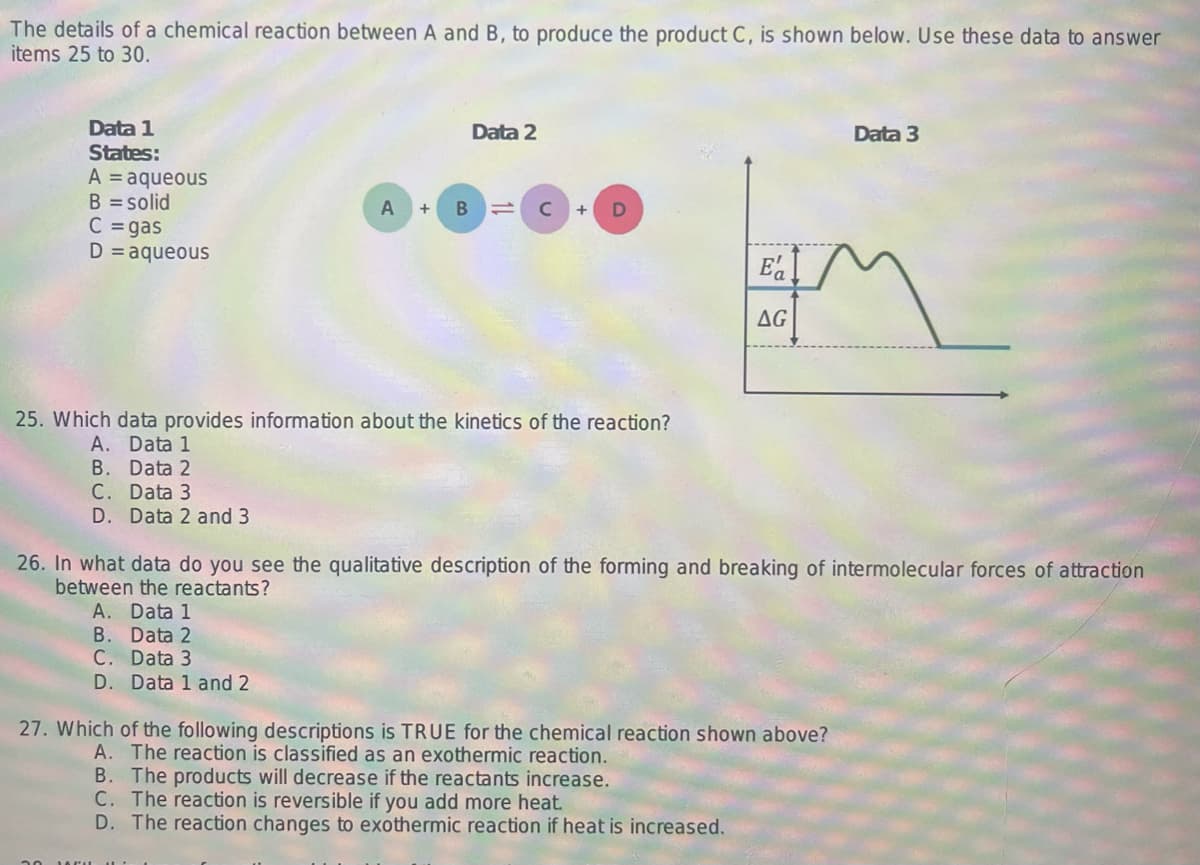
Transcribed Image Text:The details of a chemical reaction between A and B, to produce the product C, is shown below. Use these data to answer
items 25 to 30.
Data 1
States:
A = aqueous
B = solid
C = gas
D = aqueous
A
B
B.
C.
Data 2
с
D
25. Which data provides information about the kinetics of the reaction?
A. Data 1
Data 2
B.
C. Data 3
D. Data 2 and 3
Ea
AG
26. In what data do you see the qualitative description of the forming and breaking of intermolecular forces of attraction
between the reactants?
A. Data 1
Data 2
Data 3
D. Data 1 and 2
27. Which of the following descriptions is TRUE for the chemical reaction shown above?
A. The reaction is classified as an exothermic reaction.
B. The products will decrease if the reactants increase.
C. The reaction is reversible if you add more heat.
D. The reaction changes to exothermic reaction if heat is increased.
Data 3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT



EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT
