The dimensions of a room are 4.20 m × 3.00 m X 2.50 m. (a) Find the number of molecules of air in the room at at- mospheric pressure and 20.0°C. (b) Find the mass of this air, assuming that the air consists of diatomic molecules with molar mass 28.9 g/mol. (c) Find the average kinetic energy of one molecule. (d) Find the root-mean-square molecular speed. (e) On the assumption that the molar specific heat is a constant independent of temperature, we have Eint (f) What If? Find the internal energy of the air in the 5NRT/2. Find the internal energy in the air. %3| room at 25.0°C.
The dimensions of a room are 4.20 m × 3.00 m X 2.50 m. (a) Find the number of molecules of air in the room at at- mospheric pressure and 20.0°C. (b) Find the mass of this air, assuming that the air consists of diatomic molecules with molar mass 28.9 g/mol. (c) Find the average kinetic energy of one molecule. (d) Find the root-mean-square molecular speed. (e) On the assumption that the molar specific heat is a constant independent of temperature, we have Eint (f) What If? Find the internal energy of the air in the 5NRT/2. Find the internal energy in the air. %3| room at 25.0°C.
Chapter2: The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 25P: A company advertises that it delivers helium at a gauge pressure of 1.72107 Pa in a cylinder of...
Related questions
Question
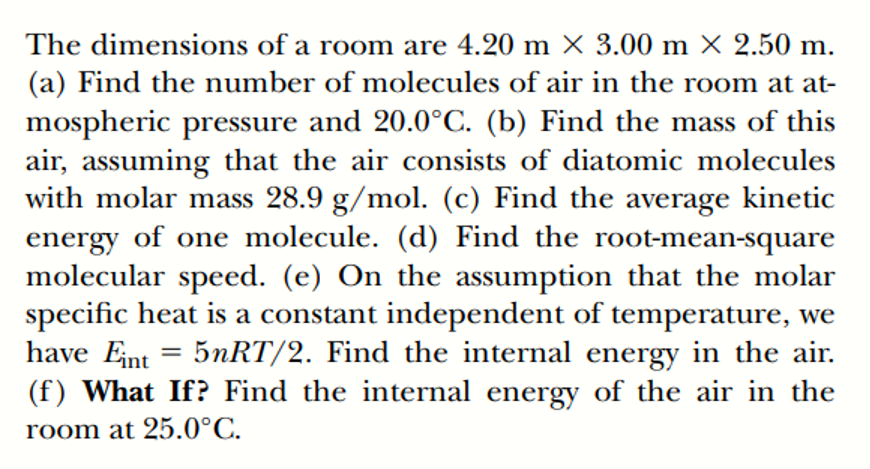
Transcribed Image Text:The dimensions of a room are 4.20 m × 3.00 m X 2.50 m.
(a) Find the number of molecules of air in the room at at-
mospheric pressure and 20.0°C. (b) Find the mass of this
air, assuming that the air consists of diatomic molecules
with molar mass 28.9 g/mol. (c) Find the average kinetic
energy of one molecule. (d) Find the root-mean-square
molecular speed. (e) On the assumption that the molar
specific heat is a constant independent of temperature, we
have Eint = 5NRT/2. Find the internal energy in the air.
(f) What If? Find the internal energy of the air in the
room at 25.0°C.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning