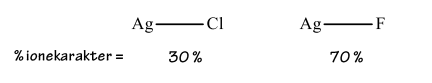
The dipole moment of gaseous Ag – Cl is ? = 6.08 D, and the bond distance is r(Ag − Cl) = 228. 1 pm. Calculate the percent ionic character for this bond in the gas phase. In solid form it is however, found the following values for the two silver halides Ag − CL and Ag − F: See image Explain why solid silver chloride is sparingly soluble in water, while solid silver fluoride is moderately soluble
The dipole moment of gaseous Ag – Cl is ? = 6.08 D, and the bond distance is r(Ag − Cl) = 228. 1 pm. Calculate the percent ionic character for this bond in the gas phase. In solid form it is however, found the following values for the two silver halides Ag − CL and Ag − F: See image
Explain why solid silver chloride is sparingly soluble in water, while solid silver fluoride is moderately soluble
B)
Explain why solid silver chloride is sparingly soluble in water, while solid silver fluoride is moderately soluble
Given extra information:
Formal charge: ?? = ??? – (??? + ???); where ??? = valence electrons in free atom, ???
= free-pair electrons, ??? = number of bonds to the atom. The elementary charge: ? = 1.6022 × 10−19 C.
1 Debye (?)= 3.3356 × 10−30 C × m. The dipole moment has definition: ? = ? × ?, where ? is partial
charge in Coulomb (?), ? is the bond distance (?). Picometer: 1 ?? = 1 × 10−12
Step by step
Solved in 7 steps









