The dissociation vapor pressure of a salt A2B(s) A2ig) + B(g) at 367°C is 208 kPa but at 477°C it has risen to 547 kPa. For the dissociation reaction of A2B(5), calculate (i) the equilibrium constant, (ii) the standard reaction Gibbs energy, (iii) the standard enthalpy, and (iv) the standard entropy of dissociation, all at 422°C. Assume that the vapor behaves as a perfect gas and that A,H° and A,S° are independent of temperature in the range given.
The dissociation vapor pressure of a salt A2B(s) A2ig) + B(g) at 367°C is 208 kPa but at 477°C it has risen to 547 kPa. For the dissociation reaction of A2B(5), calculate (i) the equilibrium constant, (ii) the standard reaction Gibbs energy, (iii) the standard enthalpy, and (iv) the standard entropy of dissociation, all at 422°C. Assume that the vapor behaves as a perfect gas and that A,H° and A,S° are independent of temperature in the range given.
Chapter10: Effect Of Electrolytes On Chemical Equilibria
Section: Chapter Questions
Problem 10.8QAP
Related questions
Question
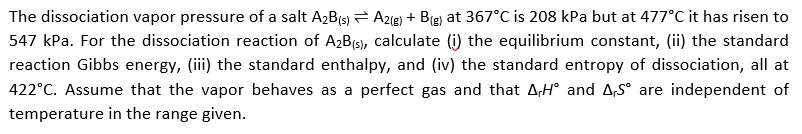
Transcribed Image Text:The dissociation vapor pressure of a salt A2B(s) A2ig) + B(g) at 367°C is 208 kPa but at 477°C it has risen to
547 kPa. For the dissociation reaction of A2B(5), calculate (i) the equilibrium constant, (ii) the standard
reaction Gibbs energy, (iii) the standard enthalpy, and (iv) the standard entropy of dissociation, all at
422°C. Assume that the vapor behaves as a perfect gas and that A,H° and A,S° are independent of
temperature in the range given.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

