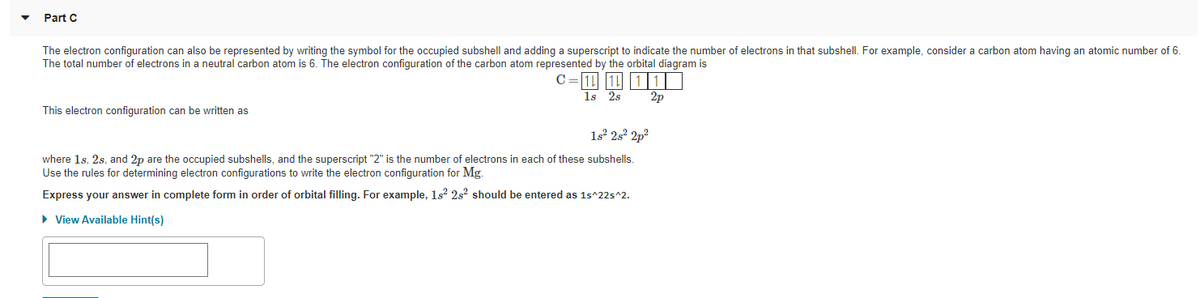
The electron configuration can also be represented by writing the symbol for the occupied subshell and adding a superscript to indicate the number of electrons in that subshell. For example, consider a carbon atom having an atomic number of 6. The total number of electrons in a neutral carbon atom is 6. The electron configuration of the carbon atom represented by the orbital diagram is 1s 2s 2p This electron configuration can be written as 1s 25° 2p² where 1s. 2s, and 2p are the occupied subshells, and the superscript "2" is the number of electrons in each of these subshells. Use the rules for determining electron configurations to write the electron configuration for Mg. Express your answer in complete form in order of orbital filling. For example, 1s 2s² should be entered as 1s^22s^2. > View Available Hint(s)
The electron configuration can also be represented by writing the symbol for the occupied subshell and adding a superscript to indicate the number of electrons in that subshell. For example, consider a carbon atom having an atomic number of 6. The total number of electrons in a neutral carbon atom is 6. The electron configuration of the carbon atom represented by the orbital diagram is 1s 2s 2p This electron configuration can be written as 1s 25° 2p² where 1s. 2s, and 2p are the occupied subshells, and the superscript "2" is the number of electrons in each of these subshells. Use the rules for determining electron configurations to write the electron configuration for Mg. Express your answer in complete form in order of orbital filling. For example, 1s 2s² should be entered as 1s^22s^2. > View Available Hint(s)
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter7: The Structure Of Atoms And Periodic Trends
Section: Chapter Questions
Problem 64GQ: Answer the questions below concerning ground state electron configurations. (a) What element has the...
Related questions
Question
Transcribed Image Text:Part C
The electron configuration can also be represented by writing the symbol for the occupied subshell and adding a superscript to indicate the number of electrons in that subshell. For example, consider a carbon atom having an atomic number of 6.
The total number of electrons in a neutral carbon atom is 6. The electron configuration of the carbon atom represented by the orbital diagram is
C
11 11 11
1s 2s
2p
This electron configuration can be written as
12 252 2p?
where 1s, 2s, and 2p are the occupied subshells, and the superscript "2" is the number of electrons in each of these subshells.
Use the rules for determining electron configurations to write the electron configuration for Mg.
Express your answer in complete form in order of orbital filling. For example, 1s? 2s? should be entered as 1s^22s^2.
• View Available Hint(s)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning