The electron configurations for four main-group elements are given. Match the electron configuration on the left with the Lewis structure on the right Clear All 122s2p323p64 3a104p5524d105p°6s х. 1s2s2p63s23p64s 3d104p524a105p* -х. 1s2s2p53s 3p6
The electron configurations for four main-group elements are given. Match the electron configuration on the left with the Lewis structure on the right Clear All 122s2p323p64 3a104p5524d105p°6s х. 1s2s2p63s23p64s 3d104p524a105p* -х. 1s2s2p53s 3p6
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter7: Covalent Bonding
Section: Chapter Questions
Problem 76QAP: In each of the following molecules, a central atom is surrounded by a total of three atoms or...
Related questions
Question
100%
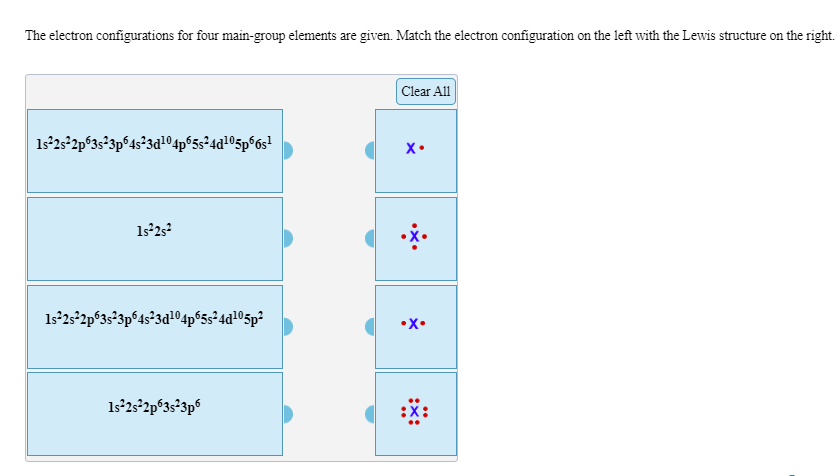
Transcribed Image Text:The electron configurations for four main-group elements are given. Match the electron configuration on the left with the Lewis structure on the right
Clear All
122s2p323p64 3a104p5524d105p°6s
х.
1s2s2p63s23p64s 3d104p524a105p*
-х.
1s2s2p53s 3p6
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning