Use the data given below to calculate the bond energies (Do) (in eV) of 'H3³CI and 'H8lBr molecules. Which bond is stronger? Molecule ve (cm¯1) De ((cm¯1) HCI 2991 53194 HBr 2649 34570
Use the data given below to calculate the bond energies (Do) (in eV) of 'H3³CI and 'H8lBr molecules. Which bond is stronger? Molecule ve (cm¯1) De ((cm¯1) HCI 2991 53194 HBr 2649 34570
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter6: Covalent Bonding
Section: Chapter Questions
Problem 81QRT: Tribromide, Br3, and triiodide, I3, ions are often found in aqueous solutions, but trifluoride ion,...
Related questions
Question
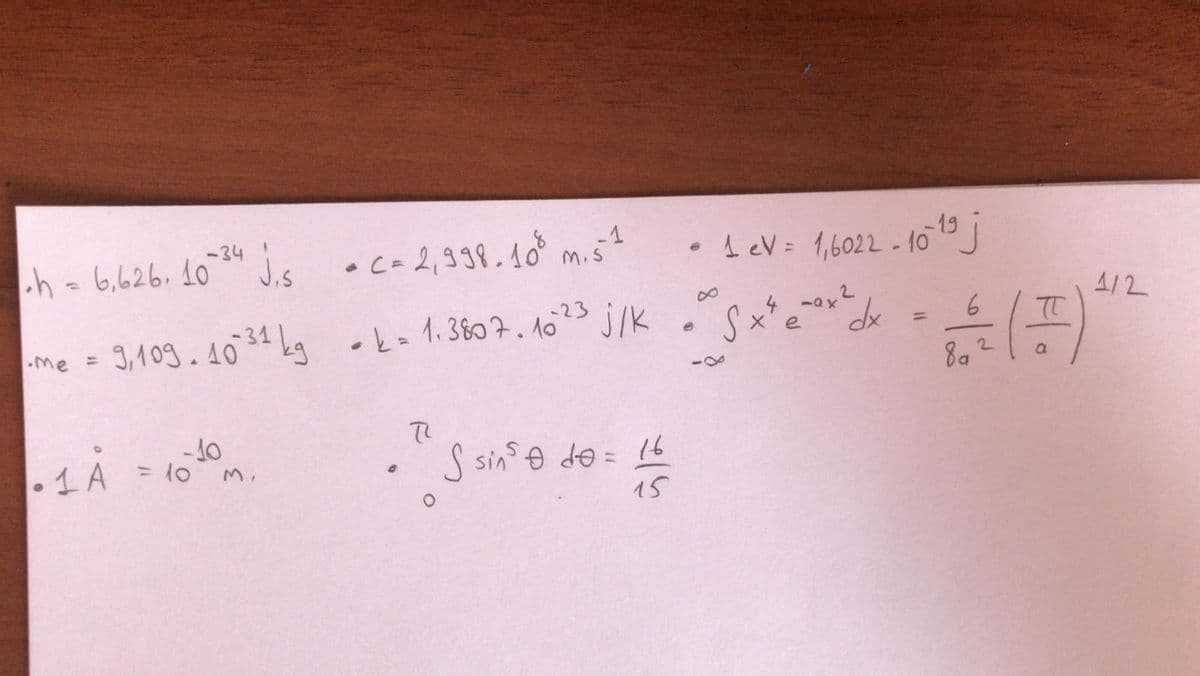
Transcribed Image Text:•c- 2,338.10 m.s?
-34
h - ** j.s
6,626. 10
•AeV= 1,6022- 10J
4/2
3,109.4034 Lg -k- 1.3807. 1023 j/K
•k=1.3807.1o
%3D
-me =
2.
-4
1 A
- 10
= 10
S sins o de = 16
%3D
15

Transcribed Image Text:Use the data given below to calculate the bond energies (Do) (in eV) of
'H³°CI and 'H®l Br molecules. Which bond is stronger?
Molecule ve (cm=1) De((cm¯1)
HCI
2991
53194
HBr
2649
34570
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning