The energy levels for the particle-in-a-box model can be used to predict the energy required to excite an electron that is part of a conjugated systems. The electron is essentially a particle that is trapped In the conjugated system. Butadiene has 4 electrons that are part of the conjugated system, which, in the lowest energy conformation, can be considered to be in two energy levels (1 and 2). H 3 H H - 133.8 pm H 133.8 pm H -H 1 H H Calculate the wavelength of light (nm) needed to promote an electron from n=2 to n=3. Hint: To first determine L, use the three bond lengths plus the distance equal to a C atom radius at each end (77 com). ) 50 210 460 600 780 145.4 pm Energy
The energy levels for the particle-in-a-box model can be used to predict the energy required to excite an electron that is part of a conjugated systems. The electron is essentially a particle that is trapped In the conjugated system. Butadiene has 4 electrons that are part of the conjugated system, which, in the lowest energy conformation, can be considered to be in two energy levels (1 and 2). H 3 H H - 133.8 pm H 133.8 pm H -H 1 H H Calculate the wavelength of light (nm) needed to promote an electron from n=2 to n=3. Hint: To first determine L, use the three bond lengths plus the distance equal to a C atom radius at each end (77 com). ) 50 210 460 600 780 145.4 pm Energy
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter11: Quantum Mechanics: Model Systems And The Hydrogen Atom
Section: Chapter Questions
Problem 11.61E: What is the physical explanation of the difference between a particle having the 3-D rotational...
Related questions
Question
6
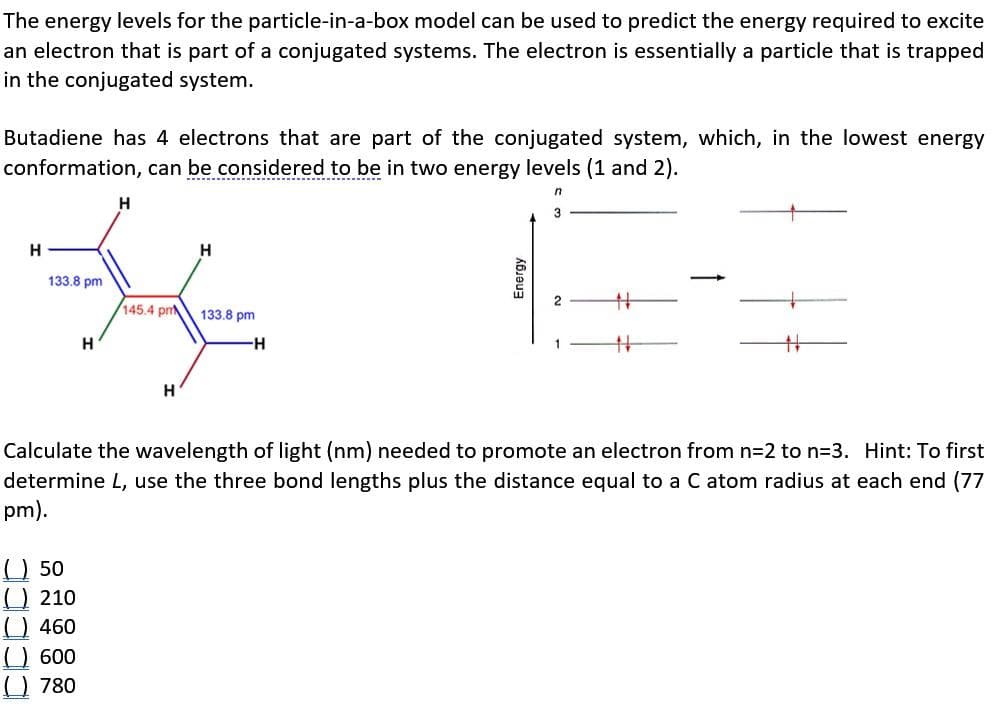
Transcribed Image Text:The energy levels for the particle-in-a-box model can be used to predict the energy required to excite
an electron that is part of a conjugated systems. The electron is essentially a particle that is trapped
in the conjugated system.
Butadiene has 4 electrons that are part of the conjugated system, which, in the lowest energy
conformation, can be considered to be in two energy levels (1 and 2).
n
H
H
H
133.8 pm
#
145.4 pm
133.8 pm
H
-H
H
#
H
Calculate the wavelength of light (nm) needed to promote an electron from n=2 to n=3. Hint: To first
determine L, use the three bond lengths plus the distance equal to a C atom radius at each end (77
pm).
(50
(210
() 460
(600
780
Energy
2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning