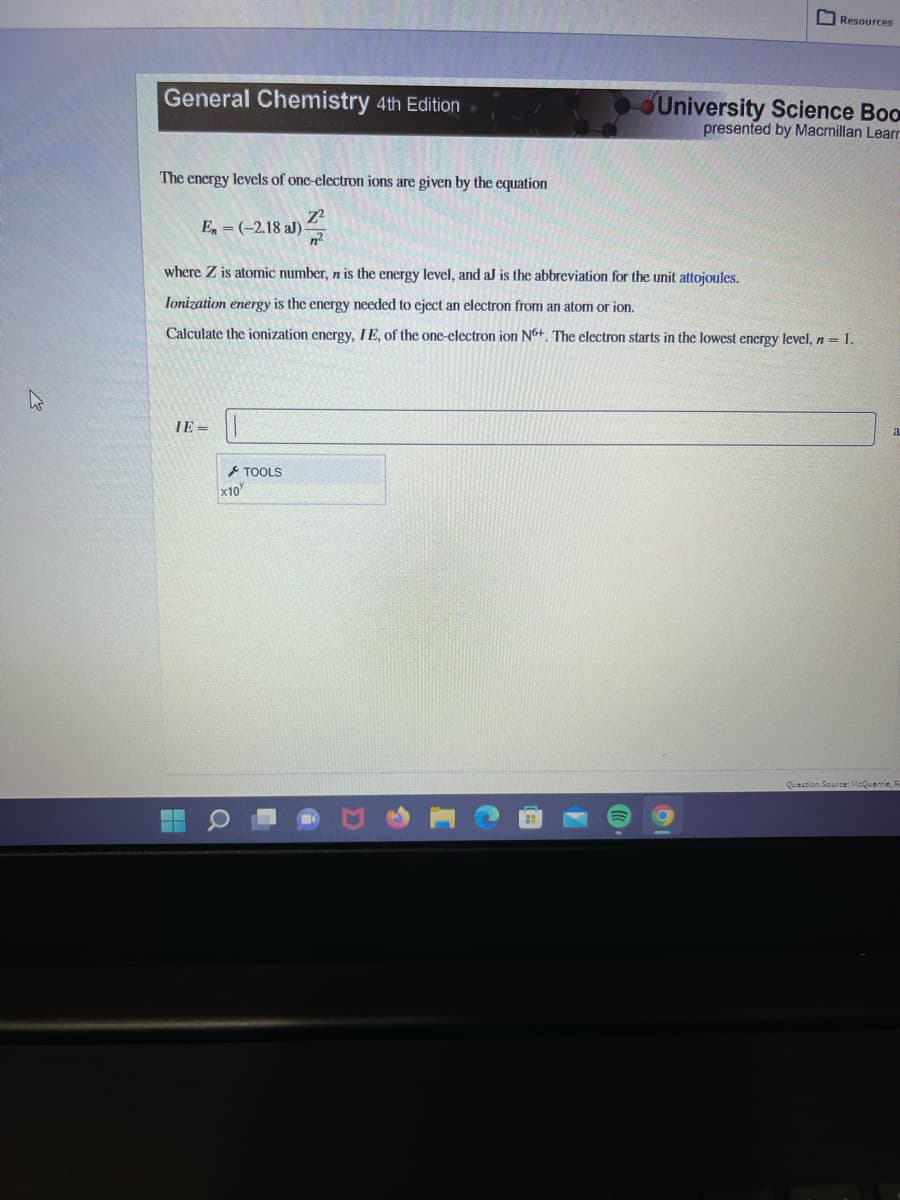
The energy levels of one-electron ions are given by the equation En = (-2.18 al) where Z is atomic number, n is the energy level, and aJ is the abbreviation for the unit attojoules. Ionization energy is the energy needed to eject an electron from an atom or ion. Calculate the ionization energy, IE, of the one-electron ion N6+. The electron starts in the lowest energy level, n = 1. IE= 2
The energy levels of one-electron ions are given by the equation En = (-2.18 al) where Z is atomic number, n is the energy level, and aJ is the abbreviation for the unit attojoules. Ionization energy is the energy needed to eject an electron from an atom or ion. Calculate the ionization energy, IE, of the one-electron ion N6+. The electron starts in the lowest energy level, n = 1. IE= 2
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter6: Electronic Structure And The Periodic Table
Section: Chapter Questions
Problem 60QAP
Related questions
Question
Arrange these elements to the atomic radius. How can we compute the ionization energy?
Transcribed Image Text:General Chemistry 4th Edition
The energy levels of one-electron ions are given by the equation
En = (-2.18 aJ)
IE=
where Z is atomic number, n is the energy level, and aJ is the abbreviation for the unit attojoules.
Ionization energy is the energy needed to eject an electron from an atom or ion.
Calculate the ionization energy, IE, of the one-electron ion N6+. The electron starts in the lowest energy level, n = 1.
x10
Resources
TOOLS
University Science Boo
presented by Macmillan Learn
a
Question Source: McQuarrie, Re
Transcribed Image Text:6
facmillan Learning
&
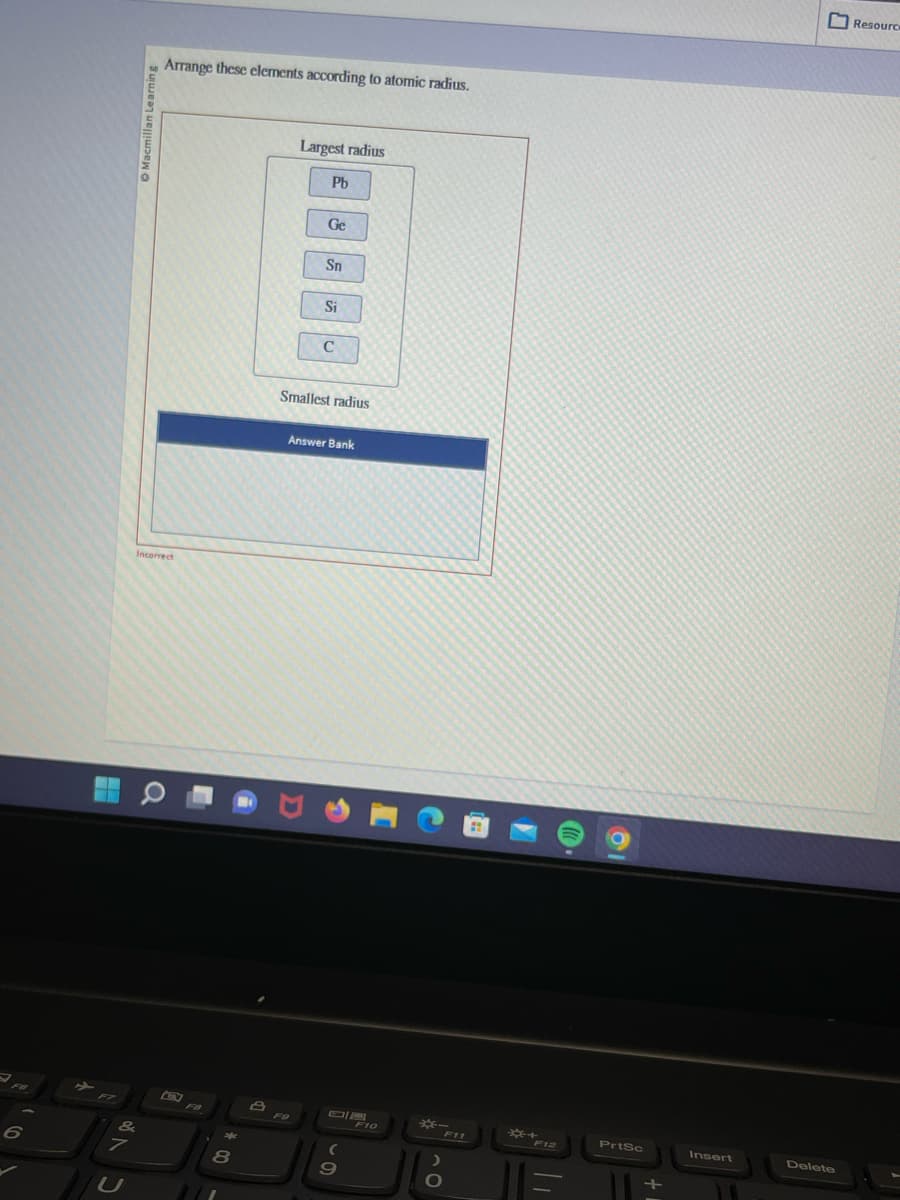
Arrange these elements according to atomic radius.
Incorrect
O
B
8
Largest radius
Pb
Ge
Sn
Si
C
Smallest radius
Answer Bank
DIE
(
9
F10
*-
>
O
*+
PrtSc
Insert
Delete
Resource
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning