The equation shows the combustion of acetylene (C₂H₂) in an arc welder. 2C₂H₂ +50₂4CO₂ + 2H₂O If 6.00 mol of acetylene undergo combustion in an excess of oxygen, how many moles of carbon dioxide can be formed? 4.80 mol O 6.00 mol O 12.0 mol 3.00 mol
The equation shows the combustion of acetylene (C₂H₂) in an arc welder. 2C₂H₂ +50₂4CO₂ + 2H₂O If 6.00 mol of acetylene undergo combustion in an excess of oxygen, how many moles of carbon dioxide can be formed? 4.80 mol O 6.00 mol O 12.0 mol 3.00 mol
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.90PAE: 4.90 Iron metal can be refined (rom the mineral hematite (Fe2O3). One way of converting the mineral...
Related questions
Question
![#
3
E
D
C
C
Essentials of General, Organic, and Biochemistry
Denise Guinn
THIRD EDITION
The equation shows the combustion of acetylene (C₂H₂) in an arc welder.
2 C₂H₂ +50₂4CO₂ + 2H₂O
If 6.00 mol of acetylene undergo combustion in an excess of oxygen, how many moles of carbon dioxide can be formed?
4.80 mol
6.00 mol
O 12.0 mol
3.00 mol
$
4
R
F
V
G Search or type URL
%
5
T
G
B
MacBook Pro
6
Y
H
*
7
N`
U
J
00
8
M
→
-
(
9
DI
K
H
<
<
I
с
L
)
O.
P
.
>
C
P
presented by Macmillan Learning
....
I
command option
4
{
[
?
1
+ 11
I
}
]
delete
return
shift](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Ff3e75a4c-de10-4a2e-a804-f32f3ae940ef%2F77c43229-a573-486a-84c7-fb62719ad1ad%2Fseu1qyb9_processed.jpeg&w=3840&q=75)
Transcribed Image Text:#
3
E
D
C
C
Essentials of General, Organic, and Biochemistry
Denise Guinn
THIRD EDITION
The equation shows the combustion of acetylene (C₂H₂) in an arc welder.
2 C₂H₂ +50₂4CO₂ + 2H₂O
If 6.00 mol of acetylene undergo combustion in an excess of oxygen, how many moles of carbon dioxide can be formed?
4.80 mol
6.00 mol
O 12.0 mol
3.00 mol
$
4
R
F
V
G Search or type URL
%
5
T
G
B
MacBook Pro
6
Y
H
*
7
N`
U
J
00
8
M
→
-
(
9
DI
K
H
<
<
I
с
L
)
O.
P
.
>
C
P
presented by Macmillan Learning
....
I
command option
4
{
[
?
1
+ 11
I
}
]
delete
return
shift
Expert Solution
Step 1
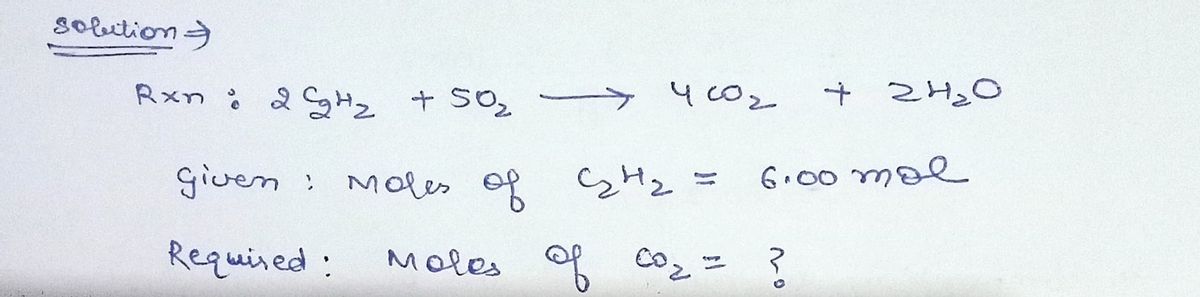
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning